Abstract
Nitric oxide (NO) induces vasodilatatory, antiaggregatory, and antiproliferative effects in vitro. To delineate potential beneficial effects of NO in preventing vascular disease in vivo, we generated transgenic mice overexpressing human erythropoietin. These animals induce polyglobulia known to be associated with a high incidence of vascular disease. Despite hematocrit levels of 80%, adult transgenic mice did not develop hypertension or thromboembolism. Endothelial NO synthase levels, NO-mediated endothelium-dependent relaxation and circulating and vascular tissue NO levels were markedly increased. Administration of the NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME) led to vasoconstriction of peripheral resistance vessels, hypertension, and death of transgenic mice, whereas wild-type siblings developed hypertension but did not show increased mortality. L-NAME-treated polyglobulic mice revealed acute left ventricular dilatation and vascular engorgement associated with pulmonary congestion and hemorrhage. In conclusion, we here unequivocally demonstrate that endothelial NO maintains normotension, prevents cardiovascular dysfunction, and critically determines survival in vivo under conditions of increased hematocrit.
The endothelium plays a key role in the regulation of blood pressure and flow (1–3). Intact endothelial cells release NO that mediates vascular relaxation in response to vasoactive substances and shear stress and provides antiproliferative and antithrombotic functions by inhibiting vascular smooth muscle cell proliferation, monocyte adhesion, platelet aggregation, and thrombosis (4–10). NO is formed from L-arginine by NO synthases (NOS). The endothelial isoform of NOS (eNOS, also termed NOS III) is the prevailing form in the vascular system (11). The aim of the present study was to delineate the role of NO in preventing vascular disease in vivo. Because polyglobulia is associated with a high incidence of arterial hypertension and thromboembolism (12, 13), we generated transgenic mice overexpressing human erythropoietin (Epo) and assessed the contribution of NO to survival and cardiovascular function in polyglobulic animals.
Materials and Methods
Generation, Characterization, and Breeding of Transgenic Mice.
A HindIII–XmnI fragment harboring the SRα promoter driving the human Epo cDNA in plasmid pTREPO (14) was replaced by the human platelet-derived growth factor (PDGF) B-chain promoter (HindIII–XmnI fragment) present in plasmid psisCAT6a (15). Subsequently, the resulting plasmid pPDGFepo was cleaved from the bacterial backbone by digestion with XmnI and NruI, and the gel-isolated fragment was purified by Qiaex (Qiagen, Chatsworth, CA) and microinjected into the pronuclei of fertilized oocytes derived from superovuled B6C3F1 hybrid mice following standard technology (16). Genomic DNA was isolated from mouse tail biopsies, digested with EcoRI, electrophoresed through 0.7% agarose, blotted onto a nylon membrane, and hybridized to a random-primed 1.5-kilobase XbaI–HindIII fragment isolated from psisCAT6a. Hematocrit, hemoglobin, and red blood cell count were determined by using standard hematological methodology, and plasma Epo levels were determined by RIA as described (17). Breeding of the resulting transgenic mouse line termed TgN(PDGFBEPO)321Zbz was performed by mating hemizygous males to wild-type females, thereby giving rise to heterozygous and wild-type littermates, the latter being used as controls. All procedures and experimental protocols were performed following the Swiss and German Animal Protection Laws and were supervised by the corresponding official Veterinary Departments.
Hemodynamics.
Blood pressure and heart rate were measured by the tail-cuff method (blood pressure recorder 8005, W + W, Münchenstein, Switzerland) in unanesthetized adult mice that underwent 4 weeks of extensive training to get used to this procedure. Mean values of three subsequent measurements were calculated. For invasive measurements, mice were anesthetized by i.p. injection of droperidol, fentanyl, and midazolam (20, 0.1, and 2 mg/kg body weight, respectively); after cannulation of the right jugular vein, anesthetics were administered continuously. Arterial pressure was measured via a catheter placed in the right carotid artery by means of a pressure transducer (Statham, Costa Mesa, CA). Cardiac output was determined by the ultrasound transit time method. Animals were ventilated with oxygen-enriched air (Minivent type 845; Hugo Sachs Elektronik, March, Germany) avoiding positive end-expiratory airway pressure (respiration rate: 130–180 breaths/min). The tidal volume was 180–230 μl. After median sternotomy, an ultrasonic flow probe was placed around the ascendant thoracic aorta below the branching of the brachiocephalic trunc and connected to a small animal blood flowmeter (T106; Transonics, Ithaca, NY). To reduce operation time, no carotid artery catheter and no jugular vein catheter were inserted.
Intravital Microscopy.
The cremaster muscle of anesthetized mice was prepared as described (18) and superfused with warm (34°C), bicarbonate-buffered (pH 7.4, pO2 ≈30 mm Hg, pCO2 ≈38 mm Hg) salt solution (in mM: 143 Na+/6 K+/2.5 Ca2+/1.2 Mg2+/128 Cl−/25 HCO3−/1.2 SO42−/1.2 H2PO4−). Twelve arterioles of different sizes were studied in each animal and observed using a microscope equipped with a closed video circuit system. Images were recorded on videotape for off-line measurement of luminal diameters after digitization (OPTIMAS, Media Cybernetics, Silver Spring, MD). Arteriolar diameters were recorded repeatedly before and after 30 min after starting the continuous local application of NG-nitro-L-arginine methyl ester (L-NAME) (3 × 10−5 M). Additionally, maximal diameters were determined by addition of a combination of different vasodilators (sodium nitroprusside, adenosine, and acetylcholine; 1 × 10−5 M each) at the end of each experiment.
Tissue Harvesting and Organ Chamber Experiments.
Upon blood collection, anesthetized mice (thiopental, 40 mg/kg body weight, i.p.) were euthanized by cervical dislocation, and organs and vessels were removed, snap-frozen in liquid nitrogen, and stored at −80°C. For Western blot analysis of NOS I, NOS II, and NOS III, the endothelium was scraped from the aorta. In addition, thoracic aortas were placed into ice-cold Krebs–Ringer bicarbonate solution (pH 7.4, 95% O2 and 5% CO2) as described (19) and dissected free from adherent connective tissue under a microscope. For isometric tension recording, aortas were cut into 3-mm rings that were placed in organ chambers containing Krebs–Ringer bicarbonate solution. After equilibration for 1 h, resting tension was gradually increased, and rings were repeatedly exposed to 0.1 M KCl until the optimal resting tension was reached. Acetylcholine concentrations were increased when contractions of the previous step were stable. Concentrations of acetylcholine, L-NAME, KCl, and sodium nitroprusside (all from Sigma, Buchs, Switzerland) are expressed as final molar concentrations in the organ chamber bath solution. After measurements, vessel rings were dried and weighed.
Measurement of Nitrate Levels in Plasma and Vascular Tissue.
Blood samples (400–500 μl) were drawn from the right ventricle and diluted 1:4 in ice-cold solution consisting of sodium chloride (0.9% wt/vol) and sodium citrate (3.8% wt/vol). After centrifugation of samples (10 min, 1,000 × g), plasma was ultrafiltered (cut off 20 kDa, 60 min, 4,000 × g). Aortic tissue (100 mg) was homogenized and subsequently diluted 1:4 in water. Quantitation of nitrate, the stable end product of NO oxidation, was performed by RP-HPLC on an ECE250/4.5 Supersil 100 RP column (Macherey & Nagel) by using ion-pairing chromatography with photodiode array detection at 210, 215, and 220 nm and related to standard curves in the 0–100 μM range generated in the same sample matrix (20). Injection volumes were 40 μl at a flow rate of 1.0 ml/min.
Statistical Analysis.
For multiple comparisons, results were analyzed with ANOVA followed by Bonferroni's correction; for comparison between two values, the unpaired Student's t test or the nonparametric Mann–Whitney test was used when appropriate. A P value <0.05 was considered significant.
Results and Discussion
Expression of human Epo cDNA in transgenic mice was driven by the human PDGF B-chain promoter that preferentially directs transgene expression to neuronal cells (15). Several resulting transgenic mouse lines showed increased Epo protein levels in the brain as measured by RIA, but plasma Epo levels were increased only in one line to about 10-fold (Table 1). As expected, elevated plasma Epo levels in hemizygous adult animals of this line enhanced erythropoiesis as reflected by the nearly doubled values for erythrocytes, hemoglobin, and hematocrit (Table 1).
Table 1.
Erythropoietic and hemodynamic parameters
| Wild type* | (n) | Transgenic* | (n) | ||
|---|---|---|---|---|---|
| Plasma Epo | Units/liter | 22.1 ± 5.2 | (14) | 259 ± 79 | (11) |
| Red blood cells | 1012/liter | 6.2 ± 0.5 | (6) | 13.8 ± 1.0 | (5) |
| Hemoglobin | g/liter | 139 ± 5 | (10) | 234 ± 13 | (10) |
| Hematocrit | % | 39.3 ± 2.7 | (8) | 79.0 ± 3.9 | (10) |
| Heart rate | Beats/min | 569 ± 18 | (17) | 605 ± 9 | (17) |
| Systolic blood pressure | mmHg | 119 ± 5 | (22) | 120 ± 5 | (22) |
| Cardiac output | ml/min | 6.4 ± 1.7 | (5) | 6.7 ± 1.7 | (8) |
Hemizygous transgenic males were mated with wild-type females, and the wild-type and transgenic siblings were analyzed at 3–6 months of age. Data are expressed as mean values ± SD for 5–22 measurements (n) as indicated.
Blood pressure is determined by cardiac output and peripheral vascular resistance. The latter depends particularly on blood viscosity, with which it bears a linear relationship over a wide range of values (21). Hematocrit is the major determinant of whole blood viscosity, which increases exponentially with hematocrit values above the physiological range. Hence, one would expect very high hematocrit levels to be associated with hypertension. However, despite hematocrit values of about 80%, blood pressure, heart rate, and cardiac output remained unaltered in transgenic adult mice when compared with their nontransgenic littermates (Table 1). Moreover, histological analysis of transgenic mice revealed no signs of myocardial infarction, stroke, or thromboembolism.
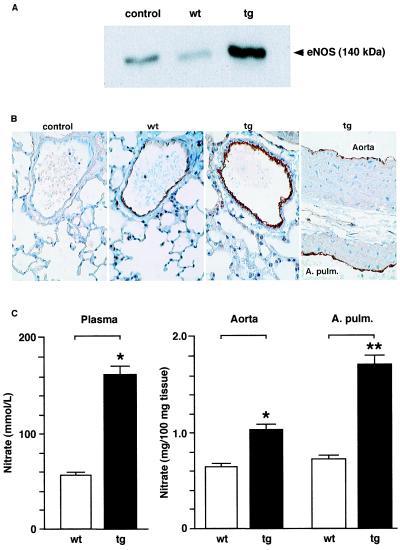
We next examined whether endothelium-derived NO provides vascular protection under conditions of high hematocrit values. Western blot analysis followed by densitometric quantitation of eNOS protein levels in transgenic and wild-type endothelium of the thoracic aorta revealed a 6-fold increase in eNOS levels in transgenic mice (Fig. 1A; n = 3). Likewise, immunohistochemical analysis showed increased eNOS expression exclusively confined to the endothelium of transgenic aorta and pulmonary arteries (Fig. 1B). Of note, neuronal (NOS I) and inducible (NOS II) NOS protein were not detectable by Western blot analysis in wild-type or transgenic endothelium (not shown).
Figure 1.
eNOS expression and NO tissue levels in wild-type (wt) and transgenic (tg) arteries. (A) Representative Western blot of eNOS protein levels in the endothelium scraped from thoracic aortas. The rabbit polyclonal antibody NOS3 (St. Cruz) was used at a 1:1000 dilution. Lysate derived from a human endothelial cell line (TransLab) was loaded as control following the supplier‘s instructions. (B) Immunochemical analysis of eNOS expression in the thoracic aorta and pulmonary arteries (A. Pulm.) of wild-type and transgenic mice using the anti-eNOS antibody described above. Note that eNOS expression is restricted to the endothelium and is markedly increased in the transgenic tissue. In the negative control, the primary antibody was omitted. (C) Quantitation of NO levels in the circulation (Left, n = 4) and in vascular tissue (Right, n = 6–7) of wild-type and transgenic mice (*, P < 0.05, **, P = 0.007).
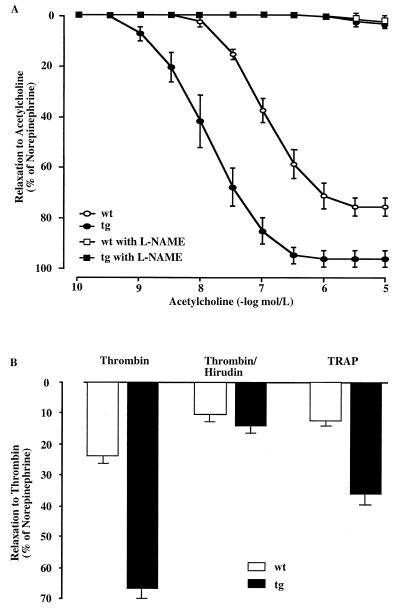
Given the multiple levels of eNOS regulation including posttranslational modifications (11, 22, 23) and an NO-dependent autoinhibitory negative feedback mechanism (24), increased expression of this enzyme does not necessarily result in elevated NO production in intact endothelial cells. Hence, it was crucial to assess NO formation in the vessel wall. Indeed, nitrate levels (the stable end product of endogenous NO oxidation) measured in the aorta and pulmonary artery of transgenic mice with high hematocrit were significantly increased compared with wild-type control organs (Fig. 1C Right). In line with this observation, plasma levels of NO were elevated 3-fold in transgenic mice (Fig. 1C Left) reflecting enhanced circulating NO bioavailability despite highly increased levels of hemoglobin, which may serve as a potential scavenger of NO in vivo (25, 26). Correspondingly, endothelium-dependent relaxations to acetylcholine were significantly augmented in the intact aorta of transgenic mice as compared with wild-type siblings (Fig. 2A), confirming increased vascular NO bioavailability. The inhibitory effect of the NOS inhibitor L-NAME (27) demonstrated that the relaxation to acetylcholine was almost exclusively mediated by endothelium-derived NO (Fig. 2A). Moreover, contraction of quiescent (e.g., non-preconstricted) aortic rings in response to L-NAME (1 × 10−9 to 3 × 10−7 M) revealed increased basal NO release (not shown). In contrast, alterations in the response of smooth muscle cells could be excluded, because endothelium-independent vasodilatation to the exogenous NO-donor sodium nitroprusside (1 × 10−10 to 3 × 10−5 M) did not differ between wild-type and transgenic rings (not shown).
Figure 2.
Endothelium-dependent relaxation of aortic rings is mediated by endothelium-derived NO. (A) Aortic rings were prepared from mice 30 ± 2 weeks old. Acetylcholine (10−10 to 3 × 10−5 M) was added in the presence or absence of the NOS inhibitor L-NAME (10−7 M) to aortic rings previously contracted with norepinephrine (70% of the response obtained with 0.1 M KCl; precontraction did not differ between wild-type and transgenic group). Compared with control littermates, endothelium-dependent relaxation of aortic rings is augmented in transgenic mice (mean ± SEM, wild type versus transgenic). Half-maximal response to acetylcholine was expressed as negative logarithm (pD2): 7.43 ± 0.1 vs. 8.36 ± 0.1 (P < 0.0001); area under the curve: 153 ± 10 vs. 283 ± 18 (P < 0.0001); maximal relaxations: −76.8 ± 3.4 vs. −97.5 ± 3.3 (P = 0.001). Note that the tissue weight of the wild-type (5.4 ± 1.7 mg) and transgenic (6.2 ± 1.9 mg) aortic rings did not differ significantly between both groups. (B) Relaxation of precontracted aortic rings upon addition of thrombin (3 × 10−8 M) alone, a combination of thrombin and hirudin (5 units/ml; note that 1 unit of hirudin neutralizes 3 × 10−8 M thrombin), or thrombin receptor agonist peptide (TRAP) (10−5 M) to the bath solution (n = 6–7).
In vivo, however, NO formation by an endogenous stimulus such as thrombin, which is predominantly generated at sites of thrombus formation, is clinically even more relevant (28). Indeed, endothelium-dependent relaxation to thrombin was also augmented in transgenic as compared with wild-type mice (Fig. 2B). This effect was blocked by the thrombin antagonist hirudin. Thrombin-induced NO release was predominantly mediated by activation of the thrombin receptor type 1, as assessed by the response to the thrombin receptor agonist peptide (TRAP), which exclusively activates this receptor (Fig. 2B). Thus, enhanced thrombin-induced NO release may prevent platelet aggregation, cell adhesion, and in turn thrombus formation in polyglobulic animals.
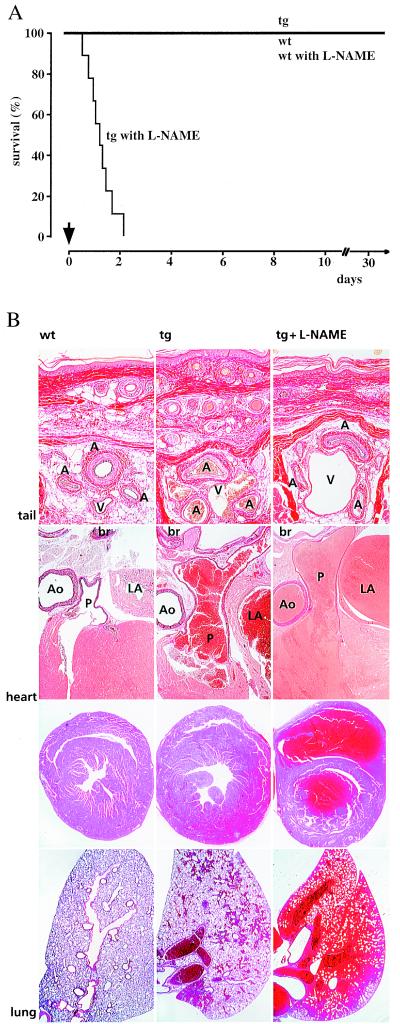
To further delineate the importance of enhanced bioavailability of NO in vivo, L-NAME was added to the drinking water of transgenic adult males and their wild-type littermates. Most strikingly, all transgenic mice died within 52 h after L-NAME application, with a mean survival time of only 33 ± 8 h (Fig. 3A). In contrast, wild-type siblings increased systolic blood pressure from 118 ± 3 mmHg to 170 ± 3 mmHg after 2 weeks of continuous ingestion of L-NAME, but appeared otherwise healthy. Histological postmortem examination of L-NAME-treated transgenic mice revealed vasoconstriction of muscular arteries of the systemic circulation, particularly in the integument (Fig. 3B). In keeping with this, morphometric analysis of these mice revealed that the inner circumference of tail arteries of L-NAME-treated animals was reduced by more than 50% compared with the untreated transgenic mice. Moreover, histological analysis revealed acute left dilation of the slightly hypertrophied heart, vascular engorgement, severe pulmonary congestion, and hemorrhage (Fig. 3B).
Figure 3.
Treatment of conscious wild-type and transgenic mice with L-NAME. (A) Drinking water supplemented or not with L-NAME at a final concentration of 0.5 g/liter was applied ad libitum to 4 to 5-month-old male transgenic mice and wild-type control littermates (n = 9–10), resulting in a calculated dosage of approximately 40 mg of L-NAME per kg body weight and day. All nine transgenic animals died within 52 h after L-NAME application, whereas mortality did not increase in wild-type siblings and nonexposed transgenic mice. The arrow indicates the start of L-NAME application. (B) Morphological analysis of tail, heart, and lung from wild-type and transgenic animals exposed or not to L-NAME. Compared with wild-type controls, transgenic mice showed enlargement and congestion of muscular arteries and great veins. Addition of L-NAME to the drinking water resulted in vasoconstriction of tail muscular arteries and further venous congestion (top row). Elastin-van Gieson staining; A, artery; V, vein (magnification, ×125). Transgenic mice showed increased diameters of pulmonary artery, pulmonary vein, and left atrium. Ingestion of L-NAME resulted in acute left ventricular dysfunction (middle rows). Elastin-van Gieson staining (second row); Ao, aorta; P, pulmonary trunc; LA, left atrium; br, broncus (×20). Hematoxylin–eosin staining (third row, ×15). Lungs of transgenic mice showed focal pulmonary hemorrhage. Exposure to L-NAME resulted in severe pulmonary congestion and massive hemorrhage (bottom row). Hematoxylin–eosin staining (×15).
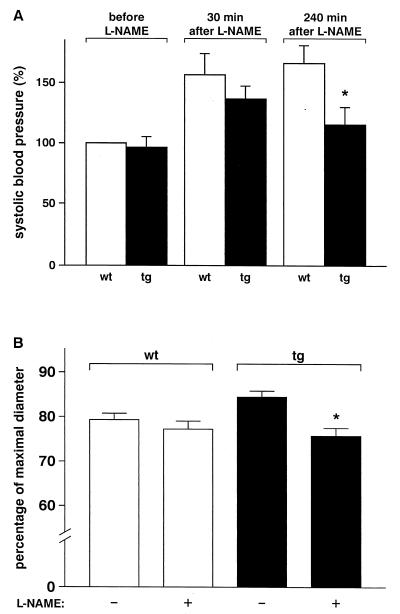
Measurement of arterial pressure in a different group of anesthetized mice revealed a peak increase of systemic blood pressure in transgenic and wild-type animals 30 min after i.v. application of the NOS inhibitor was started (Fig. 4A). However, after prolonged infusion of L-NAME, transgenic mice showed a significant decrease in blood pressure and, in analogy to the oral application of the drug, enhanced mortality. This decrease in systemic blood pressure might be partly explained by simultaneous changes in left ventricular function after NO inhibition. As histopathological examination revealed no signs of myocardial or cerebral infarction in any of the transgenic animals treated with L-NAME, this is most probably due to excessive afterload under these conditions. To assess the potential role of NO in compensating elevated resistance to flow, the effects of L-NAME on microvascular tone were determined by intravital microscopy in transgenic and wild-type animals in vivo. In arterioles of the mouse cremaster muscle, locally applied L-NAME induced significant vasoconstriction in transgenic animals only (Fig. 4B). Arteriolar tone tended to be lower in transgenic animals, an effect that was abolished by L-NAME treatment. Taken together, these data strengthen the concept of an enhanced and continuous vasodilator tone mediated by NO in arterioles in transgenic mice that, at least partially, counteracts the effect of increased viscosity on peripheral vascular resistance.
Figure 4.
Effect of L-NAME on systolic blood pressure and arteriolar diameters in anesthetized wild-type and transgenic mice. (A) Anesthetized male mice 4–5 months old (n = 6–8) received 50 mg of L-NAME per kg body weight intravenously within 15 min. Subsequently, L-NAME was infused at a dosage of 30 mg per kg per h. Three of eight transgenic mice died within 4 h after application of the NOS inhibitor was started, whereas all wild-type mice survived this procedure (*, P < 0.05). (B) Intravital microscopy of arterioles (40–100 μm; n = 14–15) was performed in anesthetized male mice 4–5 months old (n = 3 each genotype). Arteriolar diameters are depicted as a fraction of maximal diameter as determined at the end of the experiment. L-NAME (3 × 10−5 M) was applied locally to the cremaster muscle, which did not affect arterial pressure, and diameters were recorded before and 30 min after start of treatment (*, P < 0.05 vs. untreated controls).
The endothelium, by virtue of its unique location in the vessel wall, responds to shear stress, the dragging frictional force created by blood flow (2). Thus, enhanced eNOS activity in our polyglobulic animals must be due to increased blood viscosity that potentiates shear forces. Although we and others have reported that Epo exerts nonerythropoietic functions (29–33), Epo-dependent augmentation of eNOS activity by an unknown mechanism is unlikely to occur based on the recent demonstration that Epo decreases rather than increases eNOS activity in human coronary artery endothelial cells (34). Intriguingly, NO-mediated endothelial function is decreased in hypertensive patients and has been suggested to play a role in the development of Epo-induced hypertension (1, 35). Hence, the findings of the present study suggest that impairment of the L-arginine/NO pathway renders Epo-treated patients more susceptible to hypertension. This may provide the explanation why hypertension develops in some but not all patients treated with Epo.
In conclusion, we have generated a transgenic mouse line with severe polyglobulia that, due to an increased constitutive expression of eNOS associated with enhanced NO bioavailability, does not develop hypertension, stroke, myocardial infarction, or thromboembolism. The present transgenic model provides further insights into the role of endothelial factors, hematocrit-induced changes of blood flow, and the development of cardiovascular diseases such as arterial hypertension and thromboembolism.
Acknowledgments
We thank W. Kuschinsky, P. Groscurth, P. Ossent, W. Jelkmann, U. Pohl, P. Huang, J. Fandrey, and S. Ahmad for advice, discussion, and/or critically reading of the manuscript, P. Spielmann for excellent technical assistance, T. Collins for providing the PDGF B-chain promoter, and D. Blaser, M. Stäger, and F. Bootz for their assistance in maintaining the mouse colony. This work was supported by grants from the Roche Research Foundation, the Käthe Zingg-Schwichtenberg Fonds, the Stiftung für wissenschaftliche Forschung an der Universität Zürich, and the EMDO-Stiftung (all to M.G.), the Swiss National Science Foundation (to M.G., F.R., and T.F.L.), and the Deutsche Forschungsgemeinschaft (to F.R.).
Abbreviations
- Epo
erythropoietin
- NOS
nitric-oxide synthase
- eNOS
endothelial NOS
- PDGF
platelet-derived growth factor
- L-NAME
NG-nitro-L-arginine methyl ester
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Vallance P, Collier J, Moncada S. Lancet. 1989;2:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- 2.Pohl U, Holtz J, Busse R, Bassenge E. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- 3.Lüscher T F, Vanhoutte P M. The Endothelium: Modulator of Cardiovascular Function. Boca Raton, FL: CRC Press; 1990. [Google Scholar]
- 4.Furchgott R F, Zawadzki J V. Nature (London) 1980;299:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 5.Rapoport R M, Draznin M B, Murad F. Nature (London) 1983;306:174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- 6.Palmer R M, Ashton D S, Moncada S. Nature (London) 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 7.Ignarro L J, Buga G M, Wood K S, Byrns R E, Chaudhuri G. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanhoutte P M. N Engl J Med. 1988;319:512–513. doi: 10.1056/NEJM198808253190809. [DOI] [PubMed] [Google Scholar]
- 9.Panza J A, Quyyumi A A, Brush J E, Jr, Epstein S E. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 10.Huang P L, Huang Z, Mashimo H, Bloch K D, Moskowitz M A, Bevan J A, Fishman M C. Nature (London) 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 11.Michel T, Feron O. J Clin Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertinieri G, Parati G, Ulian L, Santucciu C, Massaro P, Cosentini R, Torgano G, Morganti A, Mancia G. Hypertension. 1998;31:848–853. doi: 10.1161/01.hyp.31.3.848. [DOI] [PubMed] [Google Scholar]
- 13.Hart R G, Kanter M C. Stroke. 1990;21:1111–1121. doi: 10.1161/01.str.21.8.1111. [DOI] [PubMed] [Google Scholar]
- 14.Bichet S, Wenger R H, Camenisch G, Rolfs A, Ehleben W, Porwol T, Acker H, Fandrey J, Bauer C, Gassmann M. FASEB J. 1999;13:285–295. doi: 10.1096/fasebj.13.2.285. [DOI] [PubMed] [Google Scholar]
- 15.Sasahara M, Fries J W, Raines E W, Gown A M, Westrum L E, Frosch M, Bonthron D T, Ross R, Collins T. Cell. 1991;64:217–227. doi: 10.1016/0092-8674(91)90223-l. [DOI] [PubMed] [Google Scholar]
- 16.Hergersberg M, Matsuo K, Gassmann M, Schaffner W, Luscher B, Rulicke T, Aguzzi A. Hum Mol Genet. 1995;4:359–366. doi: 10.1093/hmg/4.3.359. [DOI] [PubMed] [Google Scholar]
- 17.Wenger R H, Marti H H, Bauer C, Gassmann M. Int J Mol Med. 1998;2:317–324. doi: 10.3892/ijmm.2.3.317. [DOI] [PubMed] [Google Scholar]
- 18.de Wit C, Jahrbeck B, Schafer C, Bolz S S, Pohl U. Hypertension. 1998;31:787–794. doi: 10.1161/01.hyp.31.3.787. [DOI] [PubMed] [Google Scholar]
- 19.Lüscher T F, Diederich D, Siebenmann R, Lehmann K, Stulz P, von Segesser L, Yang Z H, Turina M, Grädel E, Weber E, et al. N Engl J Med. 1988;319:462–467. doi: 10.1056/NEJM198808253190802. [DOI] [PubMed] [Google Scholar]
- 20.Barton M, Haudenschild C C, d'Uscio L, Shaw S, Münter K, Lüscher T F. Proc Natl Acad Sci USA. 1998;95:14367–14372. doi: 10.1073/pnas.95.24.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chabanel A, Chien S. In: Hypertension: Pathophysiology, Diagnosis and Management. Laragh, Brenner, editors. New York: Raven; 1995. pp. 365–376. [Google Scholar]
- 22.Corson M, James N, Latta S, Nerem R, Berk B, Harrison D. Circ Res. 1996;79:984–991. doi: 10.1161/01.res.79.5.984. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa W C. Nature (London) 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 24.Abu-Soud H M, Wang J, Rousseau D L, Fukuto J M, Ignarro L J, Stuehr D J. J Biol Chem. 1995;270:22997–23006. doi: 10.1074/jbc.270.39.22997. [DOI] [PubMed] [Google Scholar]
- 25.Gow A J, Stamler J S. Nature (London) 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 26.Gow A J, Luchsinger B P, Pawloski J R, Singel D J, Stamler J S. Proc Natl Acad Sci USA. 1999;96:9027–9032. doi: 10.1073/pnas.96.16.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rees D D, Palmer R M, Schulz R, Hodson H F, Moncada S. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Ruschitzka F T, Rabelink T, Julmy F, Noll G, Dolezal H, Althaus U, Lüscher T F. Circulation. 1997;95:1870–1876. doi: 10.1161/01.cir.95.7.1870. [DOI] [PubMed] [Google Scholar]
- 29.Anagnostou A, Liu Z, Steiner M, Chin K, Lee E S, Kessimian N, Noguchi C T. Proc Natl Acad Sci USA. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Digicaylioglu M, Bichet S, Marti H H, Wenger R H, Rivas L A, Bauer C, Gassmann M. Proc Natl Acad Sci USA. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasuda Y, Masuda S, Chikuma M, Inoue K, Nagao M, Sasaki R. J Biol Chem. 1998;273:25381–25387. doi: 10.1074/jbc.273.39.25381. [DOI] [PubMed] [Google Scholar]
- 32.Marti H H, Wenger R H, Rivas L A, Straumann U, Digicaylioglu M, Henn V, Yonekawa Y, Bauer C, Gassmann M. Eur J Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 33.Sakanaka M, Wen T C, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X Q, Vaziri N D. Hypertension. 1999;33:894–899. doi: 10.1161/01.hyp.33.3.894. [DOI] [PubMed] [Google Scholar]
- 35.Martin J, Moncada S. Lancet. 1988;1:644. doi: 10.1016/s0140-6736(88)91438-9. [DOI] [PubMed] [Google Scholar]