Abstract
The thin layer of airway surface liquid (ASL) contains antimicrobial substances that kill the small numbers of bacteria that are constantly being deposited in the lungs. An increase in ASL salt concentration inhibits the activity of airway antimicrobial factors and may partially explain the pathogenesis of cystic fibrosis (CF). We tested the hypothesis that an osmolyte with a low transepithelial permeability may lower the ASL salt concentration, thereby enhancing innate immunity. We found that the five-carbon sugar xylitol has a low transepithelial permeability, is poorly metabolized by several bacteria, and can lower the ASL salt concentration in both CF and non-CF airway epithelia in vitro. Furthermore, in a double-blind, randomized, crossover study, xylitol sprayed for 4 days into each nostril of normal volunteers significantly decreased the number of nasal coagulase-negative Staphylococcus compared with saline control. Xylitol may be of value in decreasing ASL salt concentration and enhancing the innate antimicrobial defense at the airway surface.
A thin layer of liquid covering the airway surface (ASL) contains many antimicrobial substances, including lysozyme, lactoferrin, secretory leukoproteinase inhibitor, human β defensins 1 and 2, secretory phospholipase A2, and the cathelicidin LL-37 (for reviews see refs. 1–4). These agents acting alone and synergistically form part of the local pulmonary host defense system, killing the small numbers of bacteria that are constantly being deposited on the airway surface. The antibacterial activity of most of these agents is salt-sensitive; an increase in salt concentration inhibits the activity of individual factors and attenuates synergy between agents.
We recently proposed that disruption of this innate defense system causes, in part, the well-known propensity of cystic fibrosis (CF) airways for bacterial infection (5). CF lung disease is initially characterized by infection with a variety of bacteria, and as the disease progresses Staphylococcus aureus and Pseudomonas aeruginosa predominate (5, 6). We hypothesized that the loss of cystic fibrosis transmembrane conductance Cl− channels leads to a higher ASL salt concentration, which reduces antimicrobial potency and thereby impairs the innate immune system (7, 8). Several studies using in vitro model systems of the human airway have reported that salt concentrations are lower in ASL than in serum and are elevated in CF (8–11). However, the applicability of these observations to the in vivo human airway has not been established with certainty. Some in vivo studies have reported low NaCl concentrations in non-CF and higher values in CF ASL (12), whereas other reports have concluded that the ASL NaCl is similar to that of serum in both non-CF and CF (13, 14). The difficulty in measuring ASL electrolyte concentrations is due to its tiny volume (9, 15), to liquid application to the airway surface followed by measurements before a steady state is achieved (15), and to artifacts associated with the use of filter paper to sample ASL (16).
We hypothesized that lowering the ASL NaCl concentration could increase the activity of endogenous antimicrobials. Such an effect could be of value in preventing airway infections, irrespective of the absolute salt concentration in ASL or of differences between CF and non-CF. We considered several factors. First, the airway epithelium is water permeable (17). Consistent with this finding, when large volumes of liquid are placed on the apical surface, liquid absorption is isotonic (9, 15). Thus, if water were simply added to the airway surface, electrolyte concentrations would rapidly return to starting values. However, if an osmolyte that has a low transepithelial permeability were added to ASL, it might serve to lower the salt concentration. Somewhat analogous to this effect, the relatively impermeable osmolyte lactose allows the water-permeable mammary gland duct epithelium to maintain the luminal NaCl concentration at 5–10 mM (18). Second, an osmolyte that is nonionic would be required, because it is ionic strength that inhibits antimicrobial activity, not osmolarity (19, 20). Third, the osmolyte should not provide a ready carbon source for bacterial growth. Fourth, the osmolyte should be safe in humans. Fifth, because many endogenous antimicrobials kill very quickly, even a transient decrease in ionic strength might be effective. Finally, a small reduction in the salt concentration, perhaps only 10 mM, might be beneficial because there is no unique relationship between antimicrobial activity and ionic strength; the lower the ionic strength, the greater the bacterial killing (20–22).
A promising osmolyte for lowering ASL ionic strength is the five-carbon sugar, xylitol, which is poorly metabolized by some bacteria (23). Interestingly, when incorporated into chewing gum, xylitol is reported to prevent dental caries (24). Moreover, in chewing gum, lozenges, or syrup, xylitol decreases the incidence of acute otitis media by 20–40% (25). Therefore, we tested the hypothesis that xylitol applied to the apical surface of human airway epithelia would lower the ASL salt concentration. We also examined the effect of xylitol on bacteria in vitro and in vivo.
Materials and Methods
Human Airway Epithelia.
Airway epithelial cells were isolated from tracheal and bronchial tissue, grown on semipermeable membranes at the air-liquid interface, and studied at least 14 days after seeding when they had differentiated and developed a ciliated apical surface (26, 27). Transepithelial resistance was 588 ± 33 Ω⋅cm2 (n = 9) for non-CF and 493 ± 24 Ω⋅cm2 (n = 9) for CF epithelia.
Measurement of Xylitol Fluxes.
To the apical surface we applied 60 μl of a xylitol solution containing (in mM) 138 xylitol, 53 NaCl, 4 KCl, 29 NaHCO3, 1.2 CaCl2, 0.6 MgCl2, and 1 NaH2PO4. The osmolality of the submucosal solution was adjusted to equal that of the mucosal solution with a vapor pressure osmometer (Wescor, Logan, UT). To the apical and basolateral solutions we added 1 × 106 cpm/ml of 3H2O. The apical solution also received 1 × 106 cpm/ml of 14C-labeled xylitol (Moravek Biochemicals, Brea, CA). After incubation at 37°C for 1–12 h, both solutions were collected and the volume and xylitol concentrations were determined; the methods have been described (9).
Measurement of Liquid Absorption and Xylitol.
Liquid absorption was measured using methods similar to those previously described (9). To the apical surface we applied 60 μl of a saline solution, a xylitol solution, or a mixture of the two. The saline solution contained (in mM) 138 NaCl, 4 KCl, 29 NaHCO3, 1.2 CaCl2, 0.6 MgCl2, and 1 NaH2PO4. The xylitol solution contained (in mM) 244 xylitol, 4 KCl, 29 NaHCO3, 1.2 CaCl2, 0.6 MgCl2, and 1 NaH2PO4. The osmolality of the submucosal solution was adjusted to equal that of the mucosal (300-310 mOsm) solution. After incubation for 4 h, apical solutions were collected under mineral oil, and the volume was measured as described (9).
Measurement of ASL Cl− Concentration.
The ASL Cl− concentration was measured as described (9). To the apical surface we applied 5 μl of a saline solution or a xylitol solution. The xylitol solution contained (in mM) 290 xylitol, 1.2 CaCl2, and 0.6 MgCl2. The saline solution contained (in mM) 145 NaCl, 1.2 CaCl2, and 0.6 MgCl2. To the basolateral medium (500 μl) we added 2.5 × 104 cpm of 3H2O and 36Cl. ASL Cl− concentration and volume were measured 24 h later.
Evaluation of the Effect of Xylitol on Bacterial Growth.
Procedures were approved by the Human Subjects Review Board of the University of Iowa. Nasal lavage fluid was collected from normal volunteers. A flexible catheter (18-gauge; Jelco, Tampa, FL) was inserted into each nostril, and the area was flushed four times with 4 ml of sterile water. Cells were removed by centrifugation, and the fluid was filtered with a sequential 0.8/0.2 μm Supor Acrodisc PF (Gelman). To study the effect of xylitol on bacterial killing by endogenous antimicrobial factors, we used a luminescence assay of bacterial viability in which Escherichia coli expresses the genes from Photorhabdus luminescens (20). Bacteria (106) were incubated with 50 μl of nasal lavage fluid in serial dilutions of 300 mM xylitol or 150 mM NaCl in a 96-well plate. Luminescence was measured after incubation at 30°C for 4 h.
To test the effect of xylitol on the growth of bacteria in a carbon-starved medium, we grew P. aeruginosa, S. aureus, and coagulase-negative Staphylococcus overnight in LB medium. Bacteria were centrifuged; resuspended in M9 medium containing 100 mM of succinate, mannitol, or sucrose; and grown overnight at 37°C. The bacteria were centrifuged and resuspended in M9 medium alone, M9 medium containing 100 mM xylitol, or 100 mM indicated metabolizable sugar as a positive control and studied in midlog phase. OD was measured after 0, 1, 2, and 4 h at 600 nm.
To test the antibiotic effect of xylitol, P. aeruginosa, S. aureus, and coagulase-negative Staphylococcus were grown overnight in LB medium, centrifuged, and resuspended in LB medium with and without 100 mM xylitol. As a positive control, antibiotics with activity to each of the bacteria were added to the medium (40 μg/ml tobramycin or 40 μg/ml levofloxacin). To test the antibiotic effect of xylitol on lower concentrations of coagulase-negative Staphylococcus saprophyticus (ATCC 15305) over longer periods of time, we incubated log dilutions of bacteria (108 to 103) in the presence or absence of 100 mM xylitol and measured OD over 18 h.
To test the effect of xylitol on the growth of normal nasal flora in a carbon-starved medium, we obtained nasal swabs from three normal volunteers and inoculated the swabs into M9 medium alone, M9 with 100 mM xylitol, or LB medium. Bacteria were incubated at 37°C for 72 h, and the ODs were recorded.
Administration of Xylitol to the Nasal Mucosa.
The study was approved by the University of Iowa Institutional Review Board. Subjects were more than 18 years old and provided written informed consent. Individuals were excluded from participation if they had a seasonal allergic rhinitis or nasal polyps, or current treatment with any antibiotic, steroid, or topical intranasal preparation. Twenty-one normal healthy subjects (10 male and 11 female, ages 20–52 years) participated.
The design was a double-blind, randomized, cross-over study. Subjects were randomized to one of two groups: xylitol followed by saline or saline followed by xylitol. A culture of both anterior nares was obtained on day 0. Subjects then sprayed each nostril with a prefilled syringe of solution four times per day for 4 days. On the morning of day 5, subjects sprayed the final application into each nostril at breakfast time, then a nasal swab was obtained 2 h later. No treatment was administered for the next 7 days. Then subjects repeated the protocol with the opposite solution. The saline solution was 0.9% NaCl in water (Baxter Health Care, Mundelein, IL); the xylitol solution was 5% xylitol (304 mM) in water. The solutions (250 μl) were nebulized by using an Accuspray syringe (Becton Dickinson Pharmaceutical Systems, Franklin Lakes, NJ). Xylitol and saline syringes were identical. The mass medium diameter of the particles was ∼60 μm. It was impossible to disguise the sweet taste of the xylitol. Fifteen of the 21 subjects were able to recognize the sweet taste of the xylitol; the other six subjects could not distinguish between the solutions.
Cultures were obtained with sterile rayon swabs (Culturette Collection and Transport System; Becton Dickinson Microbiology Systems, Sparks, MD). A swab was rotated firmly five times in each nostril. Swabs were collected by the same individual throughout the study. Swabs were directly inoculated into 1 ml of PBS and vortexed for 5 s, and 50 μl was plated by using an automated spiral plater (Spiral Biotech, Bethesda, MD) onto sheep blood agar (Remel, Lenexa, KS), and mannitol salt agar (Becton Dickinson, Sparks, MD). Plates were incubated at 37°C for 24 h, and colonies of coagulase-negative Staphylococcus colonies were identified and counted by using a Cling-On Grid (Spiral Biotech). Samples were routinely sent to the Clinical Microbiology Laboratory (University of Iowa Hospitals and Clinics) to confirm the identity of the bacteria.
In a preliminary study in eight non-CF subjects, we found that the number of coagulase-negative Staphylococcus cultured from the nasal epithelium remained relatively stable over 4 days. A power analysis (28) suggested that 39 independent nostrils would be required to show a 50% difference in the reduction in coagulase-negative Staphylococcus between treatments (power of 0.84 and an f value of 0.5, assuming that the nostrils are independent). In contrast to non-CF subjects, we found that the numbers of bacteria cultured from the CF nasal surface were quite variable over time.
Results
Xylitol Permeability of Airway Epithelia.
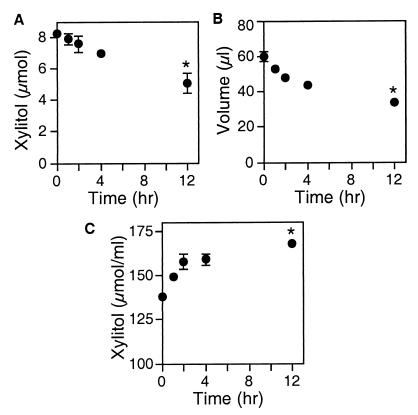
To examine the effect of xylitol on ASL, we used primary cultures of well-differentiated airway epithelia (26, 27). Because airway epithelia are water permeable (17), lowering ASL salt concentration will require an osmolyte with a relatively low transepithelial permeability. We tested the xylitol permeability by applying it to the apical surface and measuring its disappearance over time. The amount of xylitol progressively decreased; after 12 h, 40% of the applied sugar had diffused to the basolateral surface (Fig. 1). Because the volume decreased with time, the xylitol concentration increased. We obtained similar results when we measured the concentration of xylitol by NMR (not shown). Thus the xylitol permeability was not high, and the increase in concentration suggested that xylitol could temporarily hold liquid on the apical surface.
Figure 1.
Apical xylitol and volume after the addition of xylitol to the apical surface of non-CF airway epithelia. Xylitol (138 mM in 60 μl) was added to the apical surface of differentiated airway epithelia at time 0. Then, at the times indicated, the apical liquid was removed and the quantity of xylitol (A), the liquid volume (B), and the xylitol concentration (C) were determined. Data are mean ± SEM (n = 6). Some SEM bars are hidden by symbols. The asterisk indicates P < 0.01 compared with time 0.
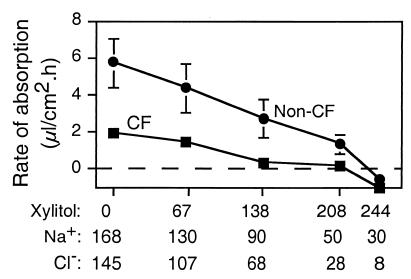
To test this hypothesis directly, we asked whether xylitol reduces the rate of liquid absorption. We applied 60 μl of a saline solution, a xylitol solution, or a mixture of the two to the apical surface. The apical solution always had the same osmolarity as the basolateral solution; thus as the xylitol concentration increased, the NaCl concentration decreased. Four hours later, we removed the liquid and determined the rate of liquid absorption. Fig. 2 shows that during a 4-h incubation with apical saline, both non-CF and CF epithelia absorbed liquid, and consistent with our previous report the rate of liquid absorption was greater in non-CF than in CF epithelia (9). In both non-CF and CF epithelia, increasing the xylitol concentration reduced the rate of liquid absorption (Fig. 2). Like the data in Fig. 1, these results indicate that xylitol is relatively nonpermeable because it reduced the absorption rate and held liquid on the apical surface.
Figure 2.
Effect of apical xylitol on the rate of liquid absorption by non-CF and CF epithelia. Sixty microliters of saline solution, xylitol solution, or indicated mixtures of the two was applied to the apical solution. Four hours later the solution was removed to measure the rate of liquid absorption. A short incubation period was chosen to avoid secondary changes in the epithelium due to the large volume of apical liquid. Data are mean ± SEM (n = 15) from three different experiments. Some SEM bars are hidden by symbols.
Xylitol Added to the Apical Surface Decreases ASL Cl− Concentration in CF Epithelia in Vitro.
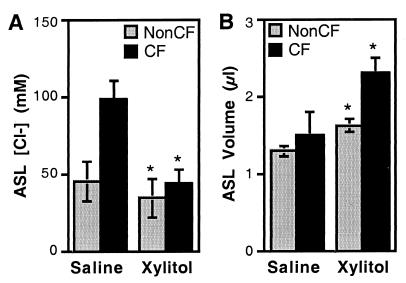
We tested the ability of xylitol to reduce the ASL salt concentration by applying a small volume (5 μl) of saline or xylitol to the apical surface. Twenty-four hours after the saline was applied (138 mM Cl−), non-CF epithelia reduced the ASL Cl− concentration to 45.3 ± 1.3 mM (Fig. 3A). This value agrees with earlier measurements of ASL Cl− concentration (9). When xylitol was applied instead of saline, the ASL Cl− concentration was even lower (34.2 ± 4.3 mM).
Figure 3.
Effect of apical xylitol on non-CF and CF ASL Cl− concentration and volume. Isosmotic xylitol or saline (5 μl) was applied to the apical surface. Twenty-four hours later, ASL Cl− concentration (A) and volume (B) were determined. The asterisk indicates a difference between the saline and the xylitol solutions (P < 0.05; n = 15–18) from three CF and three non-CF specimens.
In CF epithelia, the Cl− concentration was 98 ± 12 mM 24 h after the addition of saline (Fig. 3A). This value is approximately double that in non-CF epithelia and is consistent with our earlier measurements (9). However, with xylitol application, the Cl− concentration fell to values observed in non-CF epithelia. Xylitol also increased the estimated ASL volume in both non-CF and CF epithelia (Fig. 3B). Thus, adding xylitol to the CF epithelial surface allowed a reduction in Cl− concentration that was likely due to a combination of active transepithelial salt transport, ASL dilution, and the osmotic pressure generated by xylitol.
Xylitol Does Not Affect Bacterial Growth and Does Not Interfere with Killing by Endogenous Antimicrobial Factors.
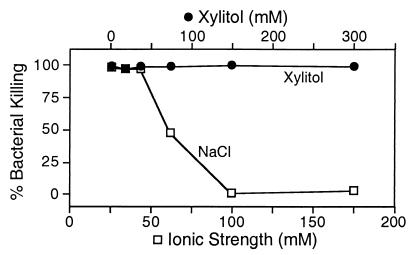
Earlier data showed that increased ionic strength, not increased osmolarity, inhibited bacterial killing by endogenous airway antimicrobial factors (20). To test the effect of xylitol, we collected nasal lavage fluid, which contains multiple antimicrobial factors, and examined killing of E. coli with a luminescence assay. Fig. 4 shows that nasal lavage fluid killed the bacteria. Although killing was inhibited as ionic strength increased, killing was not affected by an increase in xylitol concentration. We obtained similar results with P. aeruginosa and S. aureus.
Figure 4.
Effect of modifying ionic strength and xylitol concentration on killing of E. coli by nasal lavage liquid. Nasal lavage liquid was diluted with increasing concentrations of NaCl (bottom x axis) or xylitol (top x axis). SEMs are smaller than the symbols.
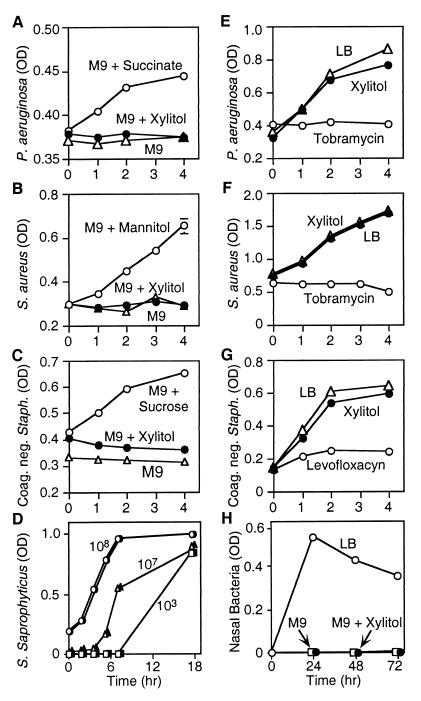
We asked whether xylitol would support the growth of the predominant colonizers in CF lungs, P. aeruginosa and S. aureus. P. aeruginosa was placed in M9 medium, which lacks a carbon source. Under these conditions, the bacteria showed no growth (Fig. 5A). Adding the energy source succinate allowed bacterial growth. In contrast, there was no growth of P. aeruginosa when M9 medium was supplemented with xylitol. Likewise, xylitol failed to support the growth of S. aureus or coagulase-negative Staphylococcus (Fig. 5 B and C). Xylitol also failed to support the growth of Staphylococcus saprophyticus, which ferments xylitol (34) (Fig. 5D). To learn whether bacteria from the nasal surface could use xylitol for growth, we obtained nasal swabs and inoculated them into medium. The bacteria grew in LB medium, whereas in M9 medium alone or M9 medium containing xylitol, there was no growth (Fig. 5H). Although xylitol did not support growth, it did not inhibit the growth of P. aeruginosa, S. aureus, or coagulase-negative Staphylococcus in rich medium (Fig. 5 E–G). As a positive control, we added a pharmaceutical antibiotic to which the bacteria were sensitive.
Figure 5.
Effect of xylitol on growth of several bacteria. Growth of P. aeruginosa (A), S. aureus (B), and coagulase-negative Staphylococcus (C) was measured as OD ▵, M9 medium alone. Xylitol (●) or succinate, mannitol, or sucrose (○) was added to M9 medium at 100 mM as indicated. (D) S. saprophyticus was grown in log phase in the presence (open symbols) and absence (closed symbols) of xylitol for 18 h. Starting bacterial concentrations were 108 cfu (circles), 107 cfu (triangles), and 103 cfu (squares). P. aeruginosa (E), S. aureus (F), and coagulase-negative Staphylococcus (G) were cultured in LB medium alone (▵), LB medium with 100 mM xylitol (●), and LB medium containing tobramycin or levofloxacin (○). (H) Nasal swabs were collected and cultured for 3 days in LB medium (○), in minimal M9 medium (□), or in M9 medium supplemented with 100 mM xylitol (●).
These results suggest that xylitol is relatively inert in terms of CF pathogens and bacteria on the nasal surface. It did not inhibit the effect of endogenous antibiotics, it did not serve as a ready carbon source for growth, and it did not have antibiotic effects of its own.
Xylitol Applied to Nasal Epithelia in Vivo Reduces the Number of Coagulase-Negative Staphylococcus.
The ability of xylitol to lower ASL Cl− concentration in vitro and its relatively inert behavior toward bacteria suggested the hypothesis that xylitol might lower ASL salt concentration, thereby enhancing bacterial killing by endogenous antimicrobial factors. However, as discussed above, methods to accurately measure ASL salt concentration in vivo remain problematic. Therefore, to test the concept, we examined the effect of xylitol administration on bacteria cultured from the nasal mucosa of normal subjects. Because pathogens such as P. aeruginosa and S. aureus are uncommon on normal nasal mucosa, we counted the number of coagulase-negative Staphylococcus, an organism commonly found on the nasal mucosa (29, 30).
We performed a randomized, double-blind, crossover study in 21 subjects. The number of coagulase-negative Staphylococcus was determined by culture of nasal swabs. Subjects then administered xylitol or a NaCl solution to both nostrils four times a day. After 4 days, the culture was repeated. After a 1-week recovery period, the study was repeated with the other treatment. The intervention (290 mM xylitol or 145 mM saline) for the first treatment was chosen at random. These agents were applied to both nostrils in 250 μl with a preloaded syringe spray device.
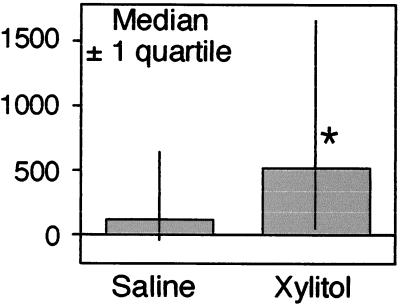
Fig. 6 shows the median change in bacterial numbers after xylitol or saline administration. ANOVA for a crossover design was applied to the change in bacterial count from pretreatment to posttreatment. The factors included in the ANOVA model were treatment, sequence of treatment, and nostril side. Before the analysis a square root transformation (sign of change times square root change) was used to normalize the data. The analysis showed that there was no significant effect of the sequence or the nostril. Thus the comparison of xylitol vs. saline was evaluated from the data of both nostrils and both sequences. The average reduction in the xylitol-treated nostrils was 597 ± 242 colony-forming units (cfu) compared with 99 ± 104 cfu for saline (P = 0.05). The median change was 500 (interquartile range of 1,152 to 120) for xylitol and 89 (interquartile range of 540 to −53) for saline (Fig. 6). Thus xylitol significantly reduced the number of coagulase-negative Staphylococcus on the nasal surface compared with saline.
Figure 6.
Effect of xylitol administration to nasal mucosa on coagulase-negative Staphylococcus. Data are the decrease in colony-forming units of coagulase-negative Staphylococcus after treatment with either saline or xylitol. Shown are median ± one quartile. The asterisk indicates P = 0.05.
Discussion
By lowering the ASL ionic strength and enhancing the effectiveness of endogenous antimicrobials, xylitol administration to the airway surface might be of value in preventing or delaying the onset of CF respiratory tract infections. Enhancing the activity of endogenous ASL antibacterial factors could have significant advantages as a preventive strategy. These factors have broad-spectrum activity against Gram-positive and Gram-negative bacteria, including the organisms that are major CF pathogens (1–4). Because many of the factors kill very quickly (some within minutes), conceivably even a transient enhancement of activity might be of value. Importantly, most bacteria, even the major CF pathogens, do not show resistance to antibacterial peptides, despite growth in the presence of subinhibitory concentrations.‡‡ In striking contrast, when currently available pharmaceutical antibiotics are administered to prevent or treat CF infections, resistance rapidly emerges (6). P. aeruginosa is notorious in this regard. Although in vitro acquired resistance to lysozyme has been reported (32), it seems likely that resistance to the mixture of endogenous factors will be uncommon, given the long period of coevolution of humans and bacteria. A recent report found that aerosolized xylitol did not significantly reduce S. pneumoniae nasal mucosal colonization in rats (33). This result is surprising because xylitol has been reported to have antimicrobial properties against this organism in vitro (34). Such results highlight the need for human studies because the ASL antimicrobial and electrolyte composition may vary significantly between species.
Although xylitol might be of value in preventing airway infections, for several reasons we think it unlikely that enhancing the activity of endogenous antimicrobials would be sufficient to treat infections once they are established. First, when chronic airway infections develop, they may exist as biofilms that are extremely resistant to antibiotics, including endogenous antimicrobial factors (35). Second, endogenous antimicrobial factors are more important in the innate immune defense against small numbers of bacteria; once infections develop, phagocytes and the acquired immune system become more important. Third, there is a significant inoculum effect, such that with large numbers of bacteria the potency of endogenous antimicrobial factors is reduced (7, 36). Fourth, in established infections, it seems possible that bacteria might develop the ability to metabolize xylitol (37). However, we do not know whether the growth of P. aeruginosa or other organisms is limited by the lack of metabolic substrate. Finally, once established, infection and inflammation alter the airway architecture, causing chronic bronchiectasis, a difficult therapeutic challenge, even in patients who do not have CF.
None of the subjects reported adverse effects of xylitol or saline. Although we have not rigorously tested for safety, we predict that xylitol should be relatively nontoxic; it is present in many foods, and it has been administered intravenously in large doses to humans (38). In addition, other agents, including hypertonic mannitol and hypertonic saline solutions, have been safely aerosolized to patients with bronchiectasis and CF to improve cough and sputum clearance (39, 40).
Previous reports indicated that xylitol could reduce the growth of α-hemolytic Streptococci, including S. pneumoniae and S. mutans; however, it had little or no effect on Hemophilus influenzae or Moraxella catarrhalis (34). Moreover, we showed that xylitol did not have antimicrobial activity on its own against coagulase-negative Staphylococcus or S. saprophyticus, yet when administered to the surface of the nasal epithelium, it decreased the number of coagulase-negative Staphylococcus. These data, plus the finding that xylitol lowered the ASL Cl− concentration in vitro, suggest that the number of nasal bacteria decreased because endogenous antimicrobial factors became more active. However, we have not measured a lower salt concentration in vivo (see above). Consequently, we cannot exclude the possibility that xylitol reduced the number of bacteria by some other mechanism. Although it is possible that mucociliary clearance was improved, we think this unlikely to be entirely responsible, because the saline solution we administered as a control had no significant effect. Nevertheless, it has been hypothesized that mucociliary clearance is defective in CF airways because of a reduced ASL volume (15). If this is the case, apical application of xylitol might be of value, because our data show that it was poorly permeable and increased ASL volume.
Earlier reports have shown that xylitol used in chewing gum, in lozenges, or as syrup reduces the risk of caries and prevents acute otitis media (24, 25). In these applications, it is possible that xylitol may have enhanced mechanical clearance of bacteria without providing an energy source. However, the mechanisms could be more complex. The mouth and oral pharynx contain endogenous antimicrobial factors (31). If xylitol administration lowers the salt concentration, the activity of those factors might increase. In the case of acute otitis media, a small decrease in the total number of bacteria at the opening of the eustachian tube might decrease the frequency of middle ear seeding and infection. Our results, plus these considerations, suggest that using xylitol to lower ASL salt concentrations could be of value for other applications, perhaps including prevention of ventilator-associated pneumonia.
In conclusion, our data suggest that xylitol delivered to the airway surface may enhance the innate antibacterial defense system. These results suggest the hypothesis that xylitol or a related osmolyte could prevent or slow the onset of bacterial infection in CF. Further studies will be required to test this hypothesis.
Acknowledgments
We thank Pary Weber, Tom Moninger, Norma Anderson, Xuihui Liu, Sam McLennan, David Welsh, and Theresa Mayhew for excellent assistance. We thank Drs. Barbara Conway, Pradeep Singh, and Sue Travis for helpful discussions. We thank Drs. Leon Burmeister and Miriam Zimmerman for the statistical analysis. We appreciate the help of the Iowa Statewide Organ Procurement Organization. We thank the University of Iowa In Vitro Cell Models Core (supported in part by the National Heart, Lung and Blood Institute; the Cystic Fibrosis Foundation; and the National Institutes of Diabetes and Digestive and Kidney Diseases) and the General Clinical Research Center (supported by RR00059). This work was supported by the National Institutes of Health (HL42385, J.Z.), the Cystic Fibrosis Foundation, and the Howard Hughes Medical Institute. J.Z. is supported by the Carver Charitable Trust. M.J.W. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- ASL
airway surface liquid
- CF
cystic fibrosis
- cfu
colony-forming units
Footnotes
Fujii, C. A., Boggs, A. F., Hurst, M. A. & Mosca, D. A. (1999) Pediatr. Pulmonol. Suppl. 19, 319 (abstr.).
References
- 1.Lehrer R I, Ganz T. Curr Opin Immunol. 1999;11:23–27. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 2.Huttner K M, Bevins C L. Pediatr Res. 1999;45:785–794. doi: 10.1203/00006450-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Bals R, Weiner D J, Wilson J M. J Clin Invest. 1999;103:303–307. doi: 10.1172/JCI6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Travis, S. M., Singh, P. K. & Welsh, M. J. (2000) Curr. Opin. Immunol., in press. [DOI] [PubMed]
- 5.Burns J L, Emerson J, Stapp J R, Yim D L, Krzewinski J, Louden L, Ramsey B W, Clausen C R. Clin Infect Dis. 1998;27:158–163. doi: 10.1086/514631. [DOI] [PubMed] [Google Scholar]
- 6.Welsh M J, Tsui L C, Boat T F, Beaudet A L. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw-Hill; 1995. pp. 3799–3876. [Google Scholar]
- 7.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. ; and erratum (1996) 87, 2. [DOI] [PubMed] [Google Scholar]
- 8.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 9.Zabner J, Smith J J, Karp P H, Widdicombe J H, Welsh M J. Mol Cell. 1998;2:397–403. doi: 10.1016/s1097-2765(00)80284-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Engelhardt J F. Am J Physiol. 1999;276:469–476. doi: 10.1152/ajpcell.1999.276.2.C469. [DOI] [PubMed] [Google Scholar]
- 11.Baconnais S, Tirouvanziam R, Zahm J-M, de Bentzmann S, Péault B, Balossier G, Puchelle E. Am J Respir Cell Mol Biol. 1999;20:605–611. doi: 10.1165/ajrcmb.20.4.3264. [DOI] [PubMed] [Google Scholar]
- 12.Joris L, Dab I, Quinton P M. Am Rev Respir Dis. 1993;148:1633–1637. doi: 10.1164/ajrccm/148.6_Pt_1.1633. [DOI] [PubMed] [Google Scholar]
- 13.Knowles M R, Robinson J M, Wood R E, Pue C A, Mentz W M, Wager G C, Gatzy J T, Boucher R C. J Clin Invest. 1997;100:2588–2595. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hull J, Skinner W, Robertson C, Phelan P. Am J Respir Crit Care Med. 1998;157:10–14. doi: 10.1164/ajrccm.157.1.9703045. [DOI] [PubMed] [Google Scholar]
- 15.Matsui H, Grubb B R, Tarran R, Randell S H, Gatzy J T, Davis C W, Boucher R C. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 16.Erjefält I, Persson C G A. Clin Exp Allergy. 1990;20:193–197. doi: 10.1111/j.1365-2222.1990.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 17.Folkesson H G, Matthay M A, Frigeri A, Verkman A S. J Clin Invest. 1996;97:664–671. doi: 10.1172/JCI118463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neville M C, Zhang P, Allen J C. In: Handbook of Milk Composition. Jensen, Robert G, editors. San Diego: Academic; 1995. pp. 577–675. [Google Scholar]
- 19.Yamauchi K, Tomita M, Giehl T J, Ellison R T. Infect Immun. 1993;61:719–728. doi: 10.1128/iai.61.2.719-728.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Travis S M, Conway B A, Zabner J, Smith J J, Anderson N N, Singh P K, Greenberg E P, Welsh M J. Am J Respir Cell Mol Biol. 1999;20:872–879. doi: 10.1165/ajrcmb.20.5.3572. [DOI] [PubMed] [Google Scholar]
- 21.Singh P K, Jia H P, Wiles K, Hesselberth J, Liu L, Conway B A, Greenberg E P, Valore E V, Welsh M J, Ganz T, et al. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson J M. J Clin Invest. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holt J G, editor. Bergey's Manual of Systemic Bacteriology. Philadelphia: Lippincott; 1984. pp. 1016–1017. [Google Scholar]
- 24.Edgar W M. Br Dent J. 1998;184:29–32. doi: 10.1038/sj.bdj.4809535. [DOI] [PubMed] [Google Scholar]
- 25.Uhari M, Kontiokari T, Niemelä M. Pediatrics. 1998;102:879–884. doi: 10.1542/peds.102.4.879. [DOI] [PubMed] [Google Scholar]
- 26.Yamaya M, Finkbeiner W E, Chun S Y, Widdicombe J H. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 27.Zabner J, Zeiher B G, Friedman E, Welsh M J. J Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 29.Citron D M, Edelstein M A C, Garcia L S, Roberts G D, Thomson R B, Washington J A. In: Bailey & Scott's Diagnostic Microbiology. Baron E J, Peterson L R, Finegold S M, editors. St. Louis: Mosby; 1994. pp. 219–233. [Google Scholar]
- 30.Woods G L, Washington J A. In: Mandell, Douglas and Bennet's Principles and Practice of Infectious Diseases. Mandell G L, Bennett J E, Dolin R, editors. Vol. 1. New York: Churchill Livingstone; 1995. pp. 169–175. [Google Scholar]
- 31.Tenovuo J, Luminkari M, Soukka T. Proc Finn Dent Soc. 1991;87:197–208. [PubMed] [Google Scholar]
- 32.Fleming A. Lancet. 1929;5501:217–220. [Google Scholar]
- 33.Kontiokari T, Svanberg M, Mattila P, Leinonen M, Uhari M. FEMS Microbiol Lett. 1999;178:313–317. doi: 10.1111/j.1574-6968.1999.tb08693.x. [DOI] [PubMed] [Google Scholar]
- 34.Kontiokari T, Uhari M, Koskela M. Antimicrob Agents Chemother. 1995;39:1820–1823. doi: 10.1128/aac.39.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh, P. K., Schaefer, A. L., Parsek, M. R., Moninger, T. O., Welsh, M. J. & Greenberg, E. P. (2000) Nature (London), in press. [DOI] [PubMed]
- 36.Thrupp L D. In: Antibiotics in Laboratory Medicine. Lorian V, editor. Baltimore: Williams & Wilkins; 1986. pp. 93–149. [Google Scholar]
- 37.Doten R C, Mortlock R P. J Bacteriol. 1985;161:529–533. doi: 10.1128/jb.161.2.529-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spitz I M, Rubenstein A H, Bersohn I, Bassler K H. Metabolism. 1970;19:24–34. doi: 10.1016/0026-0495(70)90114-9. [DOI] [PubMed] [Google Scholar]
- 39.Robinson M, Regnis J A, Bailey D L, King M, Bautovich G, Bye P T P. Am J Respir Crit Care Med. 1996;153:1503–1509. doi: 10.1164/ajrccm.153.5.8630593. [DOI] [PubMed] [Google Scholar]
- 40.Daviskas E, Anderson S D, Eberl S, Chan H-K, Bautovich G. Am J Respir Crit Care Med. 1999;159:1843–1848. doi: 10.1164/ajrccm.159.6.9809074. [DOI] [PubMed] [Google Scholar]