Abstract
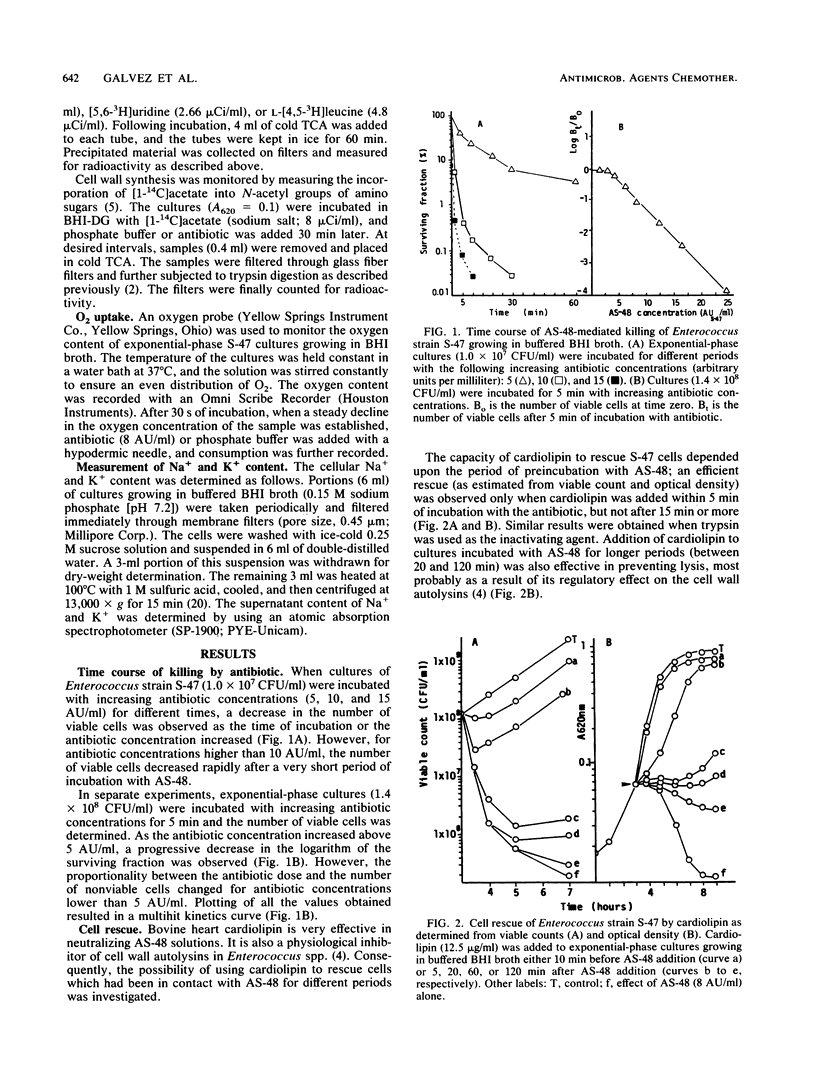
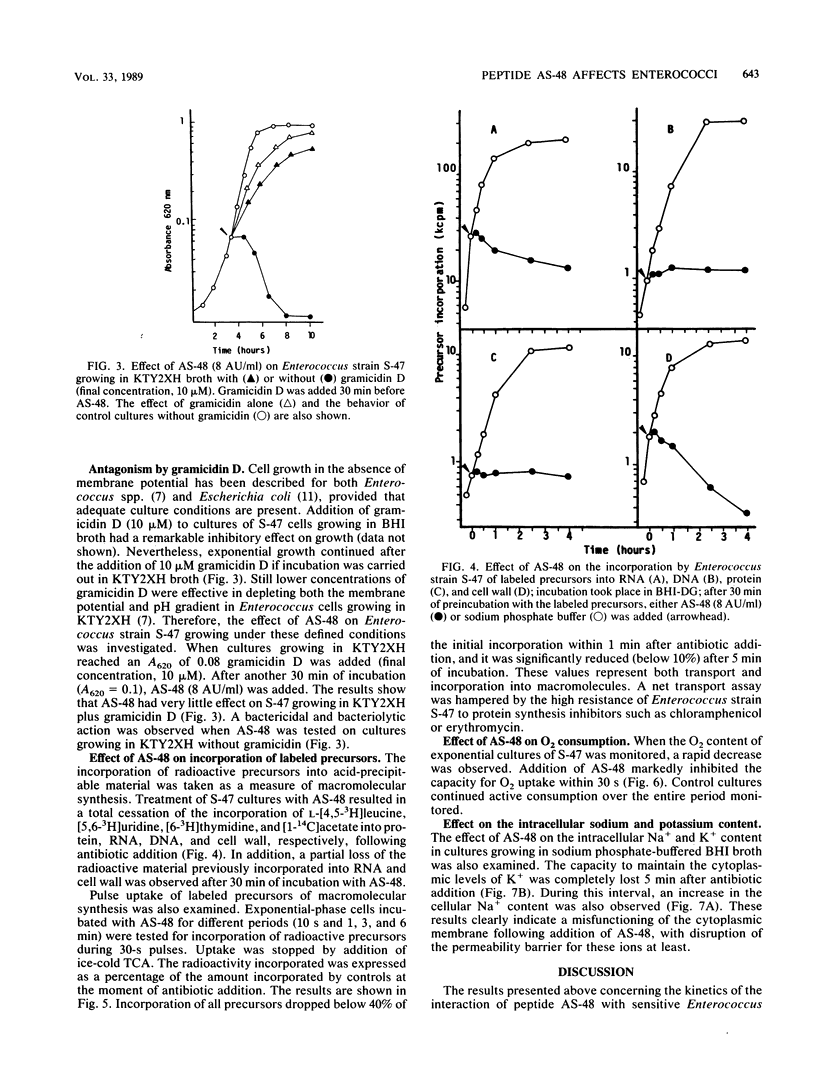
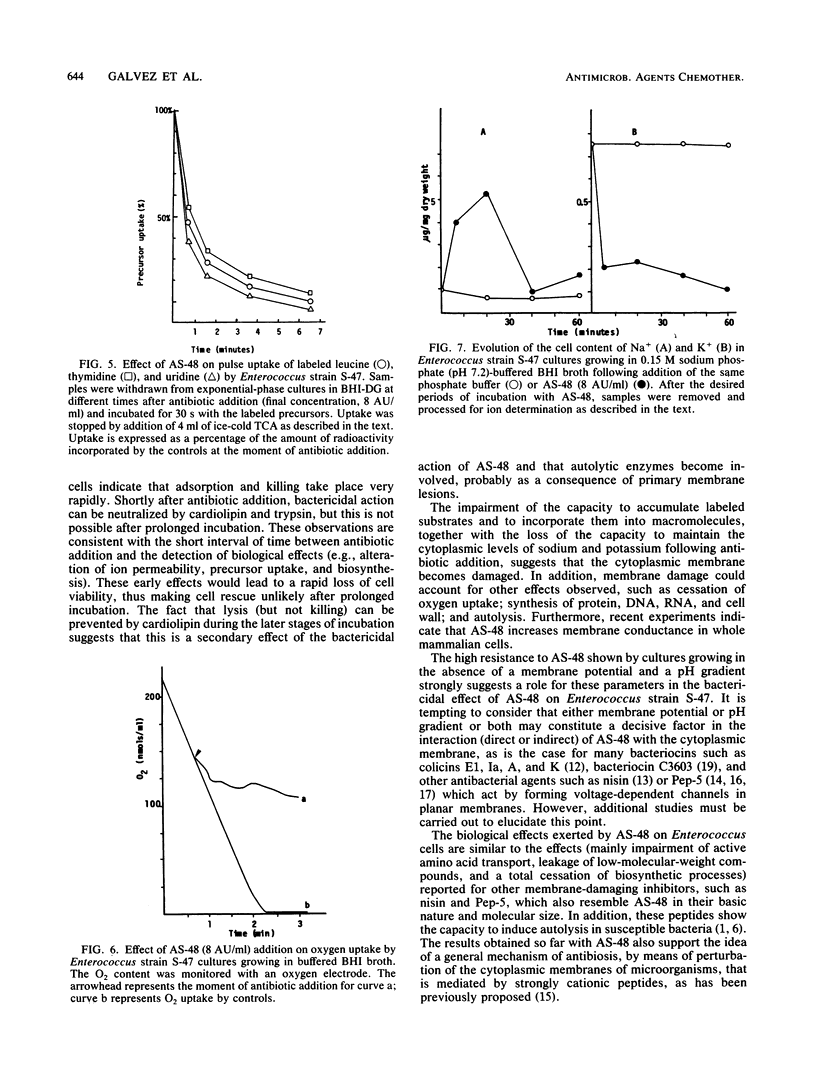
The enterococcal peptide AS-48 exerts a concentration-dependent bactericidal effect on Enterococcus faecalis subsp. liquefaciens S-47; cell rescue by cardiolipin and trypsin can be effected only in the first few minutes after antibiotic addition. Gramicidin-exposed cells are protected from killing by AS-48. Long-term and pulse incorporation of radiolabeled substrates into trichloroacetic acid-precipitable material, O2 consumption, and the ability to maintain intracellular potassium levels are impaired shortly after addition of AS-48.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK T. D., PEACHER B., PIERSON D. SURVEY OF THE BACTERIOCINES OF ENTEROCOCCI. J Bacteriol. 1963 Oct;86:702–707. doi: 10.1128/jb.86.4.702-707.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierbaum G., Sahl H. G. Induction of autolysis of staphylococci by the basic peptide antibiotics Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch Microbiol. 1985 Apr;141(3):249–254. doi: 10.1007/BF00408067. [DOI] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Shockman G. D. A rapid, guantitative, and selective estimation of radioactively labeled peptidoglycan in gram-positive bacteria. Anal Biochem. 1971 Dec;44(2):645–653. doi: 10.1016/0003-2697(71)90255-7. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Daneo-Moore L., Wicken A. J., Shockman G. D. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976 Sep;127(3):1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezélée P., Shockman G. D. Studies of the formation of peptide cross-links in the cell wall peptidoglycan of Streptococcus faecalis. J Biol Chem. 1975 Sep 10;250(17):6806–6816. [PubMed] [Google Scholar]
- Gálvez A., Maqueda M., Valdivia E., Quesada A., Montoya E. Characterization and partial purification of a broad spectrum antibiotic AS-48 produced by Streptococcus faecalis. Can J Microbiol. 1986 Oct;32(10):765–771. doi: 10.1139/m86-141. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Van Brunt J. Circulation of H+ and K+ across the plasma membrane is not obligatory for bacterial growth. Science. 1977 Jul 22;197(4301):372–373. doi: 10.1126/science.69317. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Iwanami T., Hirasawa M., Watanabe C., McGhee J. R., Shiota T. Purification and certain properties of a bacteriocin from Streptococcus mutans. Infect Immun. 1982 Mar;35(3):861–868. doi: 10.1128/iai.35.3.861-868.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita N., Unemoto T., Kobayashi H. Proton motive force is not obligatory for growth of Escherichia coli. J Bacteriol. 1984 Dec;160(3):1074–1077. doi: 10.1128/jb.160.3.1074-1077.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kékessy D. A., Piguet J. D. Bactériocinogénie et typisation de Streptococcus faecalis. Pathol Microbiol (Basel) 1971;37(2):113–121. [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl H. G. Bactericidal cationic peptides involved in bacterial antagonism and host defence. Microbiol Sci. 1985 Jul;2(7):212–217. [PubMed] [Google Scholar]
- Sahl H. G., Brandis H. Mode of action of the staphylococcin-like peptide Pep 5 and culture conditions effecting its activity. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jun;252(2):166–175. [PubMed] [Google Scholar]
- Sahl H. G. Influence of the staphylococcinlike peptide Pep 5 on membrane potential of bacterial cells and cytoplasmic membrane vesicles. J Bacteriol. 1985 May;162(2):833–836. doi: 10.1128/jb.162.2.833-836.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., McGiven A. R. Assay system for bacteriocins. Appl Microbiol. 1971 May;21(5):943–943. doi: 10.1128/am.21.5.943-943.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Ikeda T., Mitsui I., Shiota T. Mode of inhibitory action of a bacteriocin produced by Streptococcus mutans C3603. Infect Immun. 1984 May;44(2):370–378. doi: 10.1128/iai.44.2.370-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upreti G. C., Hinsdill R. D. Production and mode of action of lactocin 27: bacteriocin from a homofermentative Lactobacillus. Antimicrob Agents Chemother. 1975 Feb;7(2):139–145. doi: 10.1128/aac.7.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]


