Abstract
Protein isoprenylation is a lipid posttranslational modification required for the function of many proteins that share a carboxyl-terminal CAAX motif. The X residue determines which isoprenoid will be added to the cysteine. When X is a methionine or serine, the farnesyl-transferase transfers a farnesyl, and when X is a leucine or isoleucine, the geranygeranyl-transferase I, a geranylgeranyl group. But despite its CKVL motif, RhoB was reported to be both geranylgeranylated and farnesylated. Thus, the determinants of RhoB prenylation appear more complex than initially thought. To determine the role of RhoB CAAX motif, we designed RhoB mutants with modified CAAX sequence expressed in baculovirus-infected insect cells. We demonstrated that RhoB was prenylated as a function of the three terminal amino acids, i.e., RhoB bearing the CAIM motif of lamin B or CLLL motif of Rap1A was farnesylated or geranylgeranylated, respectively. Next, we produced a specific polyclonal antibody against farnesyl cysteine methyl ester allowing prenylation analysis avoiding the metabolic labeling restrictions. We confirmed that the unique modification of the RhoB CAAX box was sufficient to direct the RhoB distinct prenylation in mammalian cells and, inversely, that a RhoA-CKVL chimera could be alternatively prenylated. Moreover, the immunoprecipitation of endogenous RhoB from cells with the anti-farnesyl cysteine antibody suggested that wild-type RhoB is farnesylated in vivo. Taken together, our results demonstrated that the three last carboxyl amino acids are the main determinants for RhoB prenylation and described an anti-farnesyl cysteine antibody as a useful tool for understanding the cellular control of protein farnesylation.
Protein isoprenylation is a posttranslational modification by lipid recently discovered (1–3) that affects about 0.5% of cellular proteins and is essential for protein biological activity (4). Two prenyltransferases catalyze, through thioether bonds, the covalent attachment of prenyl groups from prenyl-pyrophosphates to the carboxyl-terminal cysteine of the protein included in either a CA1A2X motif (C is a cysteine, A1 and A2 usually aliphatic amino acids) or a CC, CCXX, or CXC motif (5). The CAAX protein family, which contains numerous proteins such as members of the Ras small G protein family (6–8), the nuclear lamins (9), or the γ subunit of trimeric G proteins (10–13), are prenylated by the farnesyltransferase (FTase) or geranylgeranyltransferase I (GGTase I), which transfer a 15-carbon farnesyl or a 20-carbon geranylgeranyl from the corresponding prenyl-pyrophosphate to the sulfhydryl group of the carboxyl-terminal cysteine, respectively (5). Mammalian FTase and GGTase I, which both are zinc metalloenzymes, initially were identified and purified from rat brain cytosol as α/β heterodimers (14–16). The 48-kDa α subunit is shared by both enzymes, whereas the 46-kDa β subunit of FTase is 30% identical with the 43-kDa of the GGTase I (17, 18).
It was concluded from earlier studies that the nature of the carboxyl-terminal amino acid X determines which isoprenoid (i.e., farnesyl or geranylgeranyl) will be added to the protein (15, 16). When X is a serine, methionine, cysteine, alanine, or glutamine, as in Ras or in nuclear lamins, the protein is farnesylated (14, 16, 19). When X is a leucine, isoleucine, or phenylalanine, the protein, as in the Rho/Rac family of proteins, is geranylgeranylated (15, 20). The change of carboxyl-terminal amino acid X of lamin B (from CAIM to CAIL) (15), of H-Ras (from CVLS to CVLL) (21), or of γ1 (from CVIS to CVIL) (22) is apparently sufficient to switch prenylation from farnesylation to geranylgeranylation. However, even though the prenyltransferases are quite selective for their substrates, some cross specificity has been observed. With high concentrations of peptide acceptors, both CAAX prenyltransferases were able to use either peptide as substrate, regardless of the identity of the last amino acid (15, 16). The oncoprotein K-Ras4B, whose C terminus CVIM predicts farnesylation, can be in vitro farnesylated or geranylgeranylated either by FTase or by GGTase I, respectively (23). Similar results were obtained for the Ras-related protein TC21, whose C terminus CVIF would predict geranylgeranylation (24). Moreover, when cells are treated with farnesyltransferase inhibitors in vivo, K-Ras4B as well as N-Ras are geranylgeranylated (25, 26). Likewise, the small G protein RhoB, whose CAAX box CKVL predicts geranylgeranylation, may be a substrate either of FTase and GGTase I both in vitro (27) and in vivo (28). These reports suggest that the determinants of enzyme recognition appear to be more complex than initially thought, especially for GGTase I. Nonetheless, it is still little understood how K-Ras4B or RhoB can be alternatively prenylated. It was hypothesized that the sequence responsible in RhoB and in KRas-4B should be on the NH2-terminal extremity of the CAAX box or attributable in part to the polylysine sequence immediately upstream of the CAAX box, respectively (29, 30). Recently Du et al. (31) showed that the substitution of the last 16 carboxyl-terminal residues of RhoB with the 13 carboxyl-terminal residues of RhoA directs the solely geranylgeranylation of RhoB, and proposed that geranylgeranylated RhoB might have a biological role distinct from that of farnesylated RhoB (28, 31).
The specific prenylation of the proteins is of particular interest in view of the importance of functional consequences of prenylation. Prenylation is not only necessary for membrane association but also for protein–protein interactions (5), and the nature of the linked isoprenoid can influence protein interactions, such as for trimeric G proteins and receptor (32).
To further investigate the functional consequence of simply adding different types of prenyl groups to RhoB without affecting the sequence of the mature protein, we analyzed the effect of solely substituting the CAAX box on prenylation of the RhoB protein. To characterize the added prenyl group on the mutated protein, we used not only the baculovirus system that had been demonstrated to be efficient for prenylation analysis (33) but also developed an anti-farnesyl polyclonal antibody that could recognize the farnesylated form not only of RhoB but also of H-Ras.
Materials and Methods
Plasmid Constructions.
Standard PCR mutagenesis techniques were used to generate plasmids coding for RhoB with the wild type (RhoB-CKVL), with a farnesylated (RhoB-CAIM), or a geranylgeranylated (RhoB-CLLL) CAAX sequence, or not prenylated (RhoBΔ). PCR amplifications of RhoB from pCB6-VSV-RhoB (a generous gift of B. Olofsson, Centre National de la Recherche Scientifique, Gif/Yvette, France) were done with the 5′ primer: GAAGATCTGGTACCATGGCGGCCATCCGC and the 3′ primers: CGGGATCCTCATAGCACCTTGCAGCA (RhoB-CKVL), CGGGATCCTCACATGATGGCGCAGCAGTTGATGCAGCCGTTCAG (RhoB-CAIM), CGGGATCCTCACAGCAGCAGGCAGCAGTTGATGCAGCCGTTCTG (RhoB-CLLL), or CGGGATCCTCAGCAGTTGATGCAGCCGTTCAG (RhoBΔ), respectively. The amplified fragments digested by BglII and BamHI were ligated with pCMV-intronA plasmid (a generous gift of J. Baar, University of Pittsburgh, Pittsburgh, PA) digested by BamHI and an Ires-Zeo fragment obtained from a BamHI digestion of the plasmid pUTEMCV (Cayla Société Anonyme, Toulouse, France) was added. To generate RhoA chimera with the CAAX box from RhoB, PCR amplification of RhoA from pCMV-intronA-RhoA was done with the 5′ primer AAAAACTATGTGGCAGATATCGAG and the 3′ primer TTTAGACCAACGTTCCAGAACACTCCTAGGAGATC. The amplified fragment was digested by BamHI and EcoRV and ligated in pCMV-intronA plasmid. The Bac-to-Bac baculovirus system (Life Technologies, Rockville, MD) was used to express the different RhoB forms in insect Sf9 cells. The BstYI restriction fragments containing the entire encoding sequence of RhoB with the C-terminal modifications were excised from the different pCMV-RhoB plasmids and subcloned into pFastBacHtb (containing a 6×Histidine tag) in the BamHI site.
Infection of Sf9 Cells with Baculoviruses Encoding RhoB and Purification of Recombinant Proteins.
Recombinant bacmids DNA and baculoviruses were obtained according to the manufacturer's instructions (Life Technologies). Seventy-two hours after infection, Sf9 cells were harvested and the RhoB proteins were detergent-extracted in 100 mM Tris, pH 7.4, and 2% SDS (vol/vol). Recombinant His-tagged RhoB proteins were subjected to purification by Ni2+ affinity chromatography (Ni-NTA magnetic beads; Qiagen, Chatsworth, CA).
Prenyl Group Determination.
Two days after infection with baculoviruses, Sf9 cells were labeled in growth medium containing 130 μCi/ml [5-3H]mevalonolactone (MVA) (60 Ci/mmol) (American Radiolabeled Chemicals, St. Louis), the precursor for prenylation reaction. Eighteen hours later, the Sf9 cells were harvested and homogenized in lysis buffer [100 mM Tris, pH 7.4/2% SDS (vol/vol)]. After purifying the labeled RhoB proteins and disrupting the prenyl thioether bonds with methyl iodide, the cleavage products were characterized by HPLC analysis as described (34). A gradient of solvent A (acetonitrile/water/methanol 40:40:20) to solvent B (methanol/acetonitrile 70:30) was applied for 40 min. The radioactive material was chromatographed with internal standards (such as geraniol, farnesol, and geranylgeraniol) at a flow rate of 1 ml/min.
Synthesis of S-Farnesyl l-Cysteine Methyl Ester and Coupling to Protein Carrier.
Farnesyl chloride was synthesized from trans-trans farnesol (Sigma) and N-chlorosuccinimide, as described by Davisson et al. (35). S-farnesyl l-cysteine methyl ester was prepared as described by Brown et al. (36). The 1H and 13C spectra confirmed that alkylation occurred at the sulfhydryl moiety. MS (DCI/NH3, CHCl3): m/z 340 (MH+). 13CNMR (50 MHz, CDCl3): δ (ppm): 29.9 (β cysteinyl carbon), 36.4 (C1 farnesyl).
S-farnesyl l-cysteine methyl ester (F-Cys) was conjugated to BSA and keyhole limpet hemocyanin (KLH) with a ratio of 1 mole of hapten per 50 amino acids of carrier, in the presence of 0.2% (vol/vol) glutaraldehyde (37).
Production of Antibodies Against Farnesyl-Cysteine.
Polyclonal antibodies against the farnesylated and methylated cysteine were raised in a rabbit. A solution of F-Cys KLH was emulsified with Freund's complete adjuvant, and the mixture was injected intradermally into 70 sites in the back. Boosts were given 3 wk later. The rabbit then was bled 10 days after the last injection. The immun-serum was incubated with BSA coupled on a mix of Affigel 10 and 15 (Bio-Rad), and the supernatant was purified on Affigel-F-Cys-BSA. The bound anti-farnesyl-cysteine antibodies (F-Cys Ab) were eluted with 0.2 M glycine, pH 2.2, and neutralized with 2 M Tris⋅HCl, pH 8.
Mammalian Cell Culture and Transfection.
COS-7 and NIH 3T3 cells were maintained in DMEM supplemented with 10% FCS and transfected with the different pCMV-RhoB plasmids, pCMVRhoA and pCMVRhoA/B, by using Lipofectamine Reagent plus method as indicated by the supplier (Life Technologies).
Immunoprecipitation.
For analysis of endogenous RhoB prenylation, COS-7 cells were lysed in hypotonic buffer 100 mM Tris, pH 7.5, 5 mM MgCl2, and protease inhibitors by liquid N2 and sonication and separated in cytosolic and membrane proteins by ultracentrifugation (100,000 × g for 60 min at 4°C). Membranes were resuspended in 50 mM Tris, pH 7.5, 50 mM NaCl, 5 mM MgCl2, 1% Triton X-100 (TX-100), and protease inhibitors. For the other analysis, cells were lysed with buffer containing 10 mM Tris, pH 7.4, and 2% SDS (vol/vol). Total cell lysates or membranes were delipidated with ice-cold acetone overnight. The protein pellets were solubilized in 20 mM Tris, pH 7.4, 150 mM NaCl, 1% TX-100, then clarified by microcentrifugation. Five micrograms or 75 μg of Sf9 or NIH 3T3 cell lysates, respectively, was incubated with the rabbit F-Cys Ab in 1 ml of 30 mM Hepes, pH 7.5, 10 mM NaCl, 5 mM MgCl2, 25 mM NaF, 1 mM EDTA, and 5% Nonidet P-40 (vol/vol) for 18 h. Then, 15 μl of sheep anti-rabbit IgG coupled to magnetic beads Dynabeads M-280 (Dynal, Great Neck, NY) was added. After 1 h of incubation and four washes, the immune complexes were dissociated by heating for 5 min in 150 mM Tris⋅HCl, pH 6.8, 5% SDS (wt/vol), 20% glycerol (vol/vol), 5% β-mercaptoethanol (vol/vol), and 0.01% bromophenol blue (vol/vol). One milligram of delipidated COS-7 membranes was incubated with the rabbit F-Cys Ab in 2 ml of 50 mM Tris, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% TX-100. Then, 30 μl of sheep anti-rabbit IgG coupled to magnetic beads Dynabeads M-280 was added for 18 h at 4°C. After washes, the immune complexes were dissociated for 20 min at room temperature in 150 mM Tris, pH 6.8, 6 M urea, 20% glycerol, 10 mM iodoacetamide, and 0.01% bromophenol blue.
Dot Blot and Western Blot Analysis.
Protein samples were spotted on a nitrocellulose membrane or separated by SDS/PAGE and then electroblotted onto a nitrocellulose membrane. The membranes were saturated with 5% (wt/vol) nonfat dry milk in TBST [25 mM Tris⋅HCl, pH 7.8/140 mM NaCl, 0.1% (vol/vol) Tween 20], then incubated overnight with anti-F-Cys Ab (diluted 1:1,000e), polyclonal anti-RhoB (Santa Cruz Biotechnologies), or monoclonal anti-RhoA (Santa Cruz Biotechnologies). The membranes then were washed thoroughly with TBST and incubated for 1 h with horseradish peroxidase coupled to goat anti-rabbit or anti-mouse IgG (Bio-Rad). Detection was performed with enhanced chemiluminescence kits (Amersham Pharmacia).
Results
The small GTPase RhoB has been reported to be geranylgeranylated as well as farnesylated in vivo (28) and to have distinct biological functions according to its prenylation. To characterize the functional consequence of this differential prenylation of RhoB, we generated mutants of the protein by altering the CAAX sequence to produce specific prenylation. The CKVL sequence of RhoB (Rho-CKVL) was replaced by standard PCR mutagenesis either by the CAAX box of lamin B (RhoB-CAIM), Rap1A (RhoB-CLLL), or deleted (RhoB-Δ). We showed in a parallel work using the RhoB mutants that geranylgeranylated and farnesylated forms of RhoB appeared to have similar functions in human cells in contrast with the rodent cells (38).
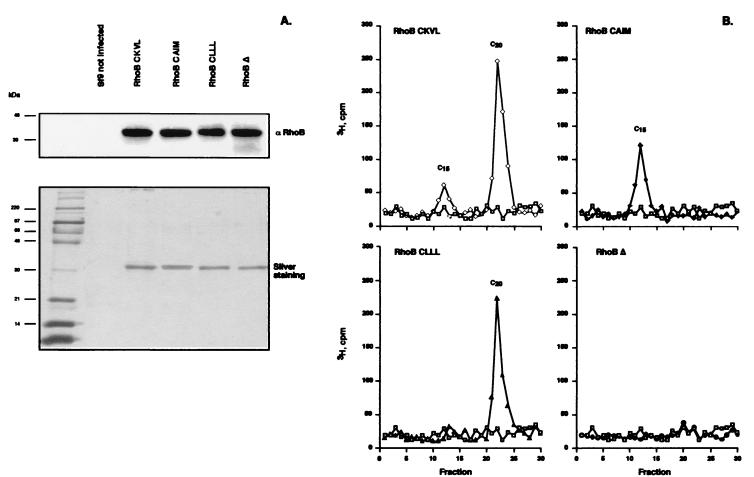
The baculovirus expression system was first used to determine whether the nature of the CAAX motif influenced the specificity of the RhoB prenylation. Expression of the different His-tagged RhoB forms in Sf9 insect cells was confirmed by Western blot after purification using Ni2+ affinity chromatography. As shown in Fig. 1, the major band obtained after elution with 250 mM imidazole, detected by SDS/PAGE and silver staining, corresponded to RhoB protein that was expressed, whatever the infection, with similar rates.
Figure 1.
The CAAX box determines the nature of the posttranslational modification. (A) His-tagged RhoB was purified from Sf9 cell homogenate on Ni-NTA magnetic beads, separated by SDS/PAGE, and analyzed by Western blot with a rabbit RhoB antibody and by silver staining. (B) The isoprenoids linked to His-tagged purified RhoB were determined after methyl iodide cleavage by reverse-phase HPLC. [3H]-Labeled isoprenoids were extracted after methyl iodide treatment and resolved by C18 reverse-phase HPLC as follows: □, Sf9 control; ◊, RhoB-CKVL; ⧫, RhoB-CAIM; ▴, RhoB-CLLL; ●, RhoB-Δ. Isoprenoids were identified by their coelution with isoprenoid standards, such as farnesol (C15) and geranylgeraniol (C20).
Analysis of the Prenylation Status of RhoB Overexpressed in Sf9 Insect Cells.
To characterize the prenyl group bound to the different mutant RhoB proteins, Sf9 cells were labeled with [3H]MVA, the precursor of prenylation reaction, after infection. After Ni2+ chromatography, the delipidated RhoB proteins were subjected to prenyl group cleavage by methyl iodide. The extracts were analyzed by reverse-phase HPLC, and the radioactivity of the collected fractions was determined. The RhoB-CKVL (wild-type protein) appeared to be prenylated either with farnesyl or geranylgeranyl. As the C20 isoprenoid would incorporate radioactivity from four MVA molecules vs. three for the C15 isoprenoid, quantitation of the radioactivity in the peaks revealed that 25% of the total RhoB protein was farnesylated. RhoB-CAIM with the CAAX box of a farnesylated protein displayed a single peak, which corresponded to farnesol. With RhoB-CLLL, bearing the CAAX box of a geranylgeranylated protein, a single peak corresponding to geranylgeraniol was observed. As expected, no radioactivity peak was observed when the CAAX box was deleted (RhoB-Δ), thus indicating that posttranslational modification did not occur on that mutant protein.
Taken together, these results implied that, in insect cells, RhoB protein with a farnesylation sequence is prenylated appropriately with a C15 isoprenoid, whereas RhoB protein with a specific geranylgeranylation sequence is solely geranylgeranylated.
Our next aim was to analyze the prenylation of RhoB mutants in mammalian cells, although certain restrictions would be encountered. Hence, after transfection, the expression of RhoB protein is much lower in mammalian cells than in insect cells (1–5% of the total proteins). Moreover, the MVA labeling requires blockage of the endogenous MVA pathway to be effective. This implies deregulation of the equilibrium of the prenylation precursors, which has been demonstrated to perturb the prenylation status (39). It is also noteworthy that only newly synthesized proteins can be characterized by this method. For all of these reasons, we decided to develop an antibody against the prenylated proteins that would provide access to a more physiological pattern of RhoB prenylation.
Characterization of the Anti-Farnesylated Cysteine Antibody.
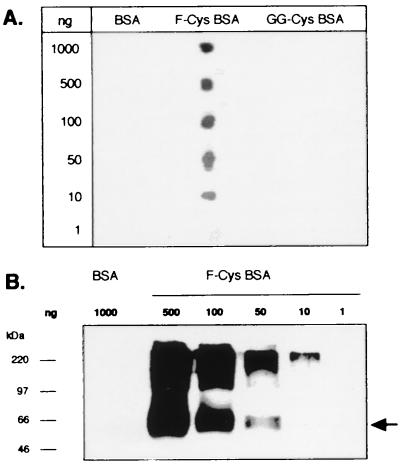
The S-farnesyl l-cysteine methyl ester was coupled to KLH (F-Cys-KLH) and injected in rabbit. After depletion on BSA of some nonspecific IgGs, the anti-F-Cys IgGs were purified on an F-Cys-BSA column, and their specificity was first determined by dot blot (Fig. 2A). Decreasing amounts of BSA, F-Cys-BSA, and BSA coupled with S-geranylgeranyl l-cysteine methyl ester (GG-Cys) were spotted on a nitrocellulose filter and probed with the anti-F-Cys Ab. The antibody did not recognize either BSA or Cys-GG-BSA, whatever the amounts deposited. In contrast, this polyclonal antibody bound to F-Cys-BSA down to 10 ng, which indicated that the antibody was highly specific to its antigen. Western blot analysis was performed to ensure whether the antibody still bound its antigen after denaturation (Fig. 2B). Different amounts of BSA or modified BSA were separated on SDS/PAGE then blotted to nitrocellulose membrane. As shown in Fig. 2B, after enhanced chemiluminescence detection, the antibody revealed a 66-kDa band only in the F-Cys-BSA lanes. The upper band should correspond to F-Cys-BSA polymers produced by glutaraldehyde coupling.
Figure 2.
Specificity of the rabbit F-Cys antibody. (A) Different amounts of each modified BSA were spotted on a nitrocellulose filter and incubated with the anti-F-Cys Ab (dilution 1:500). (B) Decreasing amounts of farnesylated BSA were analyzed by Western blot with the anti-F-Cys Ab (dilution 1:250).
Immunoprecipitation of the Farnesylated RhoB Form in Sf9 Cells.
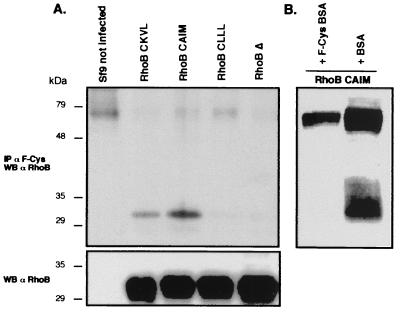
After Ni+ chromatography, the His-tagged RhoB proteins expressed by Sf9 cells were analyzed by Western blot with the anti-F-Cys Ab. Band detection was only possible in those lanes in which RhoB-CKVL and RhoB-CAIM migrated (data not shown). No signal was obtained when applied to RhoB-CLLL, RhoBΔ, or control cell lysates. But despite use of high quantities of purified RhoB (>1 μg), the Western blot with anti-F-Cys Ab was of poor quality. Therefore, we tested the ability of anti-F-Cys Ab to immunoprecipitate farnesylated RhoB. As shown in Fig. 3A, the antibody was able to immunoprecipate the farnesylated form of RhoB expressed in insect cells infected with RhoB-CKVL and RhoB-CAIM cDNAs. RhoB protein generally was observed at 28 kDa on the Western blot. In this study, the proteins expressed by insect cells were tagged with 6×histidine and the rTEV protease cleavage site, which accounts for the slower migration on SDS/PAGE (>30 kDa). It is noteworthy that the signal intensity obtained with RhoB-CKVL was weaker than with RhoB-CAIM, which is solely farnesylated, whereas the protein quantities were similar or even higher for RhoB-CKVL, as confirmed by Western blot with the anti-RhoB. This difference reflected the small proportion of farnesylated RhoB in the total RhoB protein expressed from the RhoB-CKVL plasmid.
Figure 3.
Specific immunoprecipitation of the farnesylated form of RhoB in Sf9 cells. (A) Five micrograms of delipidated proteins from Sf9 cells that had expressed the different prenylated forms of RhoB was immunoprecipitated by using 0.5 μl of the anti-F-Cys Ab and analyzed by Western blotting with a rabbit anti-RhoB. Five micrograms of Sf9 cell lysates was analyzed by Western blot using the rabbit anti-RhoB Ab (Bottom). (B) An amount of 2.5 μl of the anti-F-Cys Ab was preincubated with 1 ml of BSA or F-Cys-BSA coupled to agar beads. The supernatant was recovered and used to immunoprecipitate RhoB from RhoB-CAIM expressing Sf9 cells, as described in A.
To test the specificity of the antibody, the anti-F-Cys Ab was preincubated with BSA or F-Cys-BSA coupled to agar beads overnight. The anti-F-Cys Ab preincubated with BSA was still able to immunoprecipitate the farnesylated RhoB from Sf9 cell lysates (Fig. 3B). In contrast, the antibody preincubated with the farnesylated BSA did not precipitate the protein.
The CKVL Motif Drives Dual Prenylation of RhoB.
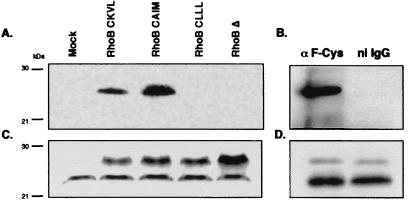
To determine whether a sole change in the CAAX motif also might influence specifically the prenylation of RhoB in mammalian systems, we then performed a similar experiment with NIH 3T3 cells. Lysates were immunoprecipitated as above with the anti-F-Cys Ab. As previously shown with insect cell extracts, the antibody only immunoprecipitated the presumably farnesylated RhoB, RhoB-CKVL, and RhoB-CAIM (Fig. 4A), whereas the RhoB protein was expressed in similar amounts, whatever the CAAX box mutation (Fig. 4C). Once again, the signal after immunoprecipitation with anti-F-Cys Ab was weaker for RhoB-CKVL than for RhoB-CAIM. These results were similar to previous observations and proved that the previously cited rule was not confined to the Sf9 cells system.
Figure 4.
Specific immunoprecipitation of the farnesylated form of RhoB in NIH 3T3 and COS-7 cells. (A) Seventy-five micrograms of proteins of NIH 3T3 cells transfected by the different prenylated forms of RhoB was immunoprecipitated with 1 μl of the anti-F-Cys Ab and analyzed by Western blot probed with a rabbit anti-RhoB Ab. (B) One milligram of delipidated membrane proteins of COS-7 cells was immunoprecipitated with nonimmune rabbit IgG (ni IgG) or anti-F-Cys Ab and analyzed by Western blot probed with a rabbit RhoB Ab. (C and D) Ten micrograms of NIH 3T3 lysates or COS-7 membranes, respectively, was analyzed by Western blot using the rabbit anti-RhoB Ab.
These results showed that overexpressed RhoB might be farnesylated in vivo. But an important question to ask is whether endogenous RhoB is also dually prenylated. As indicated in Fig. 4B, the anti-F-Cys Ab was able to precipitate endogenous RhoB in COS-7 cells, whereas the preimmune serum did not, indicating that RhoB at endogenous levels in vivo might exist as a farnesylated protein.
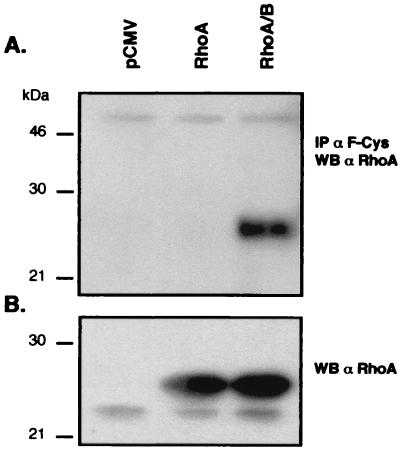
These results suggested that the CKVL motif is a main determinant for dual prenylation of RhoB. We tested this hypothesis by examining the prenylation of a RhoA chimera bearing the CAAX box of RhoB. RhoA and RhoA-CKVL chimera (RhoA/B) were transiently expressed in COS-7 cells. Cell lysates were immunoprecipitated with anti-F-Cys Ab as described earlier, then analyzed by Western blotting with an anti-RhoA antibody. As shown in Fig. 5, the anti-F-Cys Ab is able to precipitate RhoA-CKVL but not RhoA, indicating that RhoA-CKVL could be farnesylated.
Figure 5.
Analysis of the prenylation of RhoA-CKVL chimera expressed in COS-7 cells. (A) A total of 150 μg of proteins from COS-7 cells transfected either by pCMV, pCMV-RhoA, or pCMV-RhoA/B was immunoprecipitated with the rabbit anti-F-Cys Ab and analyzed by Western blot probed with a mouse RhoA Ab. (B) Ten micrograms of cell lysates was analyzed by Western blot using the mouse anti-RhoA Ab.
Discussion
Although protein prenylation specificity, whether farnesylation or geranylgeranylation, was largely observed, some proteins, i.e., K-Ras4B or RhoB, could be alternatively prenylated. Despite its CKVL sequence, the small GTPase RhoB has been described to be either farnesylated or geranylgeranylated in vivo (28, 29). This observation is of physiological importance because Lebowitz et al. (28, 31) suggested that RhoB might have distinct cellular roles, depending on the nature of prenylation. However, the biochemical parameters controlling this differential prenylation of protein are still not understood. The data presented here demonstrate that modification of the three carboxyl-terminal amino acids of the RhoB protein is sufficient to induce a specific prenylation. Moreover, for this work, we produced a polyclonal antibody against the farnesyl cysteine methyl ester, which allowed characterization of the farnesylation status of a protein in its cellular environment.
Until recently, it has been acknowledged that the carboxyl-terminal amino acid of the native protein determines the nature of the prenylation reaction (5). Notwithstanding, it was reported that the prenyltransferases displayed a more complex behavior with some proteins, such as RhoB. We hypothesized that the determinants of dual RhoB prenylation could be restricted to the CAAX box, so its substitution with the CAAX box of a specifically prenylated protein would result in specific prenylation. We generated mutants with the CAAX box of lamin B (CAIM) or Rap1A (CLLL), which were respectively farnesylated or geranylgeranylated. Prenyl group analysis of the RhoB mutants expressed in insect cells showed that the protein was properly prenylated as a function of alteration of the CAAX box. Infection of Sf9 cells with a recombinant baculovirus encoding RhoB-CAIM resulted in farnesylation of the protein, whereas substitution with the CLLL sequence resulted in geranylgeranylation. These data indicate that sole modification of the CAAX sequence is sufficient to lead to the in vivo specific prenylation of RhoB protein in Sf9 cells. The prenyl group analysis of RhoB-CKVL expressed in insect cells indicated that 25% of the protein is farnesylated; but, it had been previously demonstrated that RhoB-CKVL bore a significantly higher proportion of the farnesyl group (28, 29). This divergence might be because of the use of a different cell system and labeling conditions. Herein, we used the baculovirus expressing system described as convenient for posttranslational modification analysis (40). The insect cells were radiolabeled with the precursor of the farnesyl-pyrosphosphate and geranylgeranyl-pyrosphosphate groups, using [3H]MVA, as a radioactive label. Other laboratories have used mammalian COS-7 cells that they radiolabeled with [3H]MVA in the presence of inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase, the enzyme that catalyses the synthesis of MVA, to reduce the pool of endogenous MVA and increase the specific radioactivity. However, it was demonstrated that a differential prenylation of proteins should occur as a function of mevalonate concentration in Chinese hamster ovary cells, and, notably, farnesylation was favored in the presence of the lowest concentration of MVA (39).
Thus, to preclude this kind of restriction in assessing the prenylation of RhoB in mammalian cells, we produced a polyclonal antibody that specifically recognizes the farnesyl group linked to a methylated cysteine. We demonstrated that this antibody was truly specific to farnesylated cysteine and did not cross react with geranylgeranylated cysteine, despite the similarity of the two antigens. We next validated its specificity on insect cell-expressed RhoB mutants, in which prenylation had previously been characterized as discussed above. The antibody was able to distinguish the different prenylated forms of RhoB and did not bind the unprenylated form of the protein. It seemed, however, better to work it on native rather than denatured proteins. Indeed probably because of the conformation of the prenyl group on nitrocellulose, it did not exhibit good sensitivity in Western blot, whereas it worked very well in immunoprecipitation. The antibody allowed us to demonstrate that, without any perturbation of the MVA pathway, overexpressed RhoB in NIH 3T3 mammalian cells was still alternatively prenylated. But, it is possible that high concentrations of protein might lead to nonspecific processing errors not seen at endogenous expression levels and that the presence of mixed prenylated RhoB therefore might be artefactual. But, by immunoprecipitating RhoB with the specific anti-farnesyl antibody, we demonstrated that endogenous wild-type RhoB could be farnesylated in vivo. Taken together, our results indicated that the CKVL sequence is sufficient for the alternative modification. This assumption was corroborated by the fact that RhoA bearing the CKVL carboxy-terminal motif appeared to be farnesylated.
In parallel, the fact that the proportion of immunoprecipitated RhoB obtained from cells expressing RhoB-CAIM is larger than from cells expressing RhoB-CKVL indicated that RhoB-CAIM should be exclusively farnesylated in mammalian cells. Furthermore, the lack of immunoprecipitation of RhoB from cells transfected with RhoB-CLLL showed that RhoB-CLLL was exclusively geranylgeranylated. As in Sf9 insect cells, the substitution of the CAAX box is sufficient to drive specific prenylation of RhoB. A comparable specific change of prenylation was observed when the CAAX box of the farnesylated H-Ras was substituted from CVLS to CLVL (21) or when X of the farnesylated Gγ1 was changed from Ser to Leu or that of the geranylgeranylated Gγ2 from Leu to Ser (22). Adamson et al. (29), however, showed that substitution of the RhoB CAAX box from CKVL to CLVL, the RhoA CAAX sequence, a specifically geranylgeranylated protein, did not impair dual prenylation of the protein. This difference might be explained by the combination of the CAAX sequence and the sequence upstream from the prenylated cysteine. One can suggest that a CLVL motif, without the upstream polylysine region, is no longer a specific geranygeranylation site. In contrast, as we have shown with the RhoA-CKVL chimera, a CKVL sequence might dictate alternative irrespective of prenylation, whatever the upstream sequence of the protein. Besides, our results might suggest that CLLL is a more specific substrate for GGTase I than CLVL, whatever the overall sequence of the protein. Our data suggest that alternative prenylation could be programmed solely by the last four CAAX amino acids but did not exclude that additional determinants may be present.
It is noteworthy that the RhoB-CLLL and RhoB-CAIM mutants allow the synthesis of RhoB proteins that are, after complete processing (i.e., proteolysis of the three carboxyl-terminal amino acids, carboxymethylation, and palmitoylation), identical to the mature wild-type farnesylated and geranylgeranylated RhoB. Taken together, our results point out the importance of our cellular models expressing RhoB under specific prenylation when testing the role of the prenyl group in its physiological functions and in the induced signalization pathway. Moreover, the anti-F-Cys antibody was proved to specifically immunoprecipitate, the farnesylated form of ectopic overexpressed H-Ras, from NIH 3T3 cells (data not shown), as well as small amounts of endogenous farnesylated RhoB, indicating that it could be a useful tool for analyzing the farnesylation status of many prenylated proteins even when they are present at low copy number in vivo.
Our results outline the importance of the role of the three carboxyl-terminal amino acids of RhoB as a determinant of specific prenylation. In addition, they describe an anti-farnesyl antibody that represents a technical breakthrough, opening the way to investigations into the cellular control of protein farnesylation.
Acknowledgments
We thank Dr. B. Olofsson and Dr. J. Baar for providing us with pCB6-VSV-RhoB and pCMV-intronA plasmids, respectively. We thank Yvan Boublick and Lionel Lourenço Dias for their helpful technical assistance in the Sf9 culture and infection and in cloning the RhoA-CKVL chimera. This work was supported by the French Ministère de l'Enseignement Supérieur et de la Recherche, grants from the Groupe de Recherche de l'Institut Claudius Regaud, and the Ligue Nationale de Lutte contre le Cancer, Région Midi-Pyrénées.
Abbreviations
- FTase
farnesyltransferase
- GGTase
geranylgeranyltransferase
- F-Cys
S-farnesyl l-cysteine methyl ester
- KLH
keyhole limpet hemocyanin
- MVA
mevalonolactone
References
- 1.Schmidt R A, Schneider C J, Glomset J A. J Biol Chem. 1984;259:10175–10180. [PubMed] [Google Scholar]
- 2.Sinensky M, Logel J. Proc Natl Acad Sci USA. 1985;82:3257–3261. doi: 10.1073/pnas.82.10.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maltese W A, Sheridan K M. J Cell Physiol. 1987;133:471–481. doi: 10.1002/jcp.1041330307. [DOI] [PubMed] [Google Scholar]
- 4.Epstein W, Lever D, Leining L, Bruenger E, Rilling H. Proc Natl Acad Sci USA. 1991;88:9668–9670. doi: 10.1073/pnas.88.21.9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang F L, Casey P J. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 6.Deschenes R J, Stimmel J B, Clarke S, Stock J, Broach J R. J Biol Chem. 1989;264:11865–11873. [PubMed] [Google Scholar]
- 7.Casey P J, Solski P A, Der C J, Buss J E. Proc Natl Acad Sci USA. 1989;86:8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buss J E, Quilliam L A, Kato K, Casey P J, Solski P A, Wong G, Clark R, McCormick F, Bokoch G M, Der C J. Mol Cell Biol. 1991;11:1523–1530. doi: 10.1128/mcb.11.3.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farnsworth C C, Wolda S L, Gelb M H, Glomset J A. J Biol Chem. 1989;264:20422–20429. [PMC free article] [PubMed] [Google Scholar]
- 10.Mumby S M, Casey P J, Gilman A G, Gutowski S, Sternweis P C. Proc Natl Acad Sci USA. 1990;87:5873–5877. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamane H K, Farnsworth C C, Xie H Y, Howald W, Fung B K, Clarke S, Gelb M H, Glomset J A. Proc Natl Acad Sci USA. 1990;87:5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai R K, Perez-Sala D, Canada F J, Rando R R. Proc Natl Acad Sci USA. 1990;87:7673–7677. doi: 10.1073/pnas.87.19.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukada Y, Takao T, Ohguro H, Yoshizawa T, Akino T, Shimonishi Y. Nature (London) 1990;346:658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- 14.Reiss Y, Goldstein J L, Seabra M C, Casey P J, Brown M S. Cell. 1990;62:81–88. doi: 10.1016/0092-8674(90)90242-7. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama K, Goodwin G W, Ghomashchi F, Glomset J A, Gelb M H. Proc Natl Acad Sci USA. 1991;88:5302–5306. doi: 10.1073/pnas.88.12.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moores S L, Schabber M D, Mosser S D, Rands E, O'Hara M B, Garsky V M, Marshall M S, Pompalliano D L, Gibbs J B. J Biol Chem. 1991;266:14603–14610. [PubMed] [Google Scholar]
- 17.Ashby M N. Curr Opin Lipidol. 1998;9:99–102. doi: 10.1097/00041433-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Casey P J, Seabra M C. J Biol Chem. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 19.Reiss Y, Stradley S J, Gierasch L M, Brown M S, Goldstein J L. Proc Natl Acad Sci USA. 1991;88:732–736. doi: 10.1073/pnas.88.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoyama K, Gelb M H. J Biol Chem. 1993;268:4055–4060. [PubMed] [Google Scholar]
- 21.James G L, Brown M S, Cobb M H, Goldstein J L. J Biol Chem. 1994;269:27705–27714. [PubMed] [Google Scholar]
- 22.Lindorfer M A, Sherman N E, Woodfork K A, Fletcher J E, Hunt D F, Garrison J C. J Biol Chem. 1996;271:18582–18587. doi: 10.1074/jbc.271.31.18582. [DOI] [PubMed] [Google Scholar]
- 23.James G L, Goldstein J L, Brown M S. J Biol Chem. 1995;270:6221–6226. doi: 10.1074/jbc.270.11.6221. [DOI] [PubMed] [Google Scholar]
- 24.Carboni J M, Yan N, Cox A D, Bustelo X, Graham S M, Lynch M J, Weinmann R, Seizinger B R, Der C J, Barbacid M, Manne V. Oncogene. 1995;10:1905–1913. [PubMed] [Google Scholar]
- 25.Rowell C A, Kowalczyk J J, Lewis M D, Garcia A M. J Biol Chem. 1997;272:14093–14097. doi: 10.1074/jbc.272.22.14093. [DOI] [PubMed] [Google Scholar]
- 26.Whyte D B, Kirschmeier P, Hockenberry T N, Nunez-Oliva I, James L, Catino J J, Bishop W R, Pai J K. J Biol Chem. 1997;272:14459–14464. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong S A, Hannah V C, Goldstein J L, Brown M S. J Biol Chem. 1995;270:7864–7868. doi: 10.1074/jbc.270.14.7864. [DOI] [PubMed] [Google Scholar]
- 28.Lebowitz P F, Casey P J, Prendergast G C, Thissen J A. J Biol Chem. 1997;272:15591–15594. doi: 10.1074/jbc.272.25.15591. [DOI] [PubMed] [Google Scholar]
- 29.Adamson P, Marshall C J, Hall A, Tilbrook P A. J Biol Chem. 1992;267:20033–20038. [PubMed] [Google Scholar]
- 30.Dubyak G R, Kertesy S B. Arch Biochem Biophys. 1997;341:129–139. doi: 10.1006/abbi.1997.9946. [DOI] [PubMed] [Google Scholar]
- 31.Du W, Lebowitz P F, Prendergast G C. Mol Cell Biol. 1999;19:1831–1840. doi: 10.1128/mcb.19.3.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kisselev O, Ermolaeva M, Gautam N. J Biol Chem. 1995;270:25356–25358. doi: 10.1074/jbc.270.43.25356. [DOI] [PubMed] [Google Scholar]
- 33.Kalman V K, Erdman R A, Maltese W A, Robishaw J D. J Biol Chem. 1995;270:14835–14841. doi: 10.1074/jbc.270.24.14835. [DOI] [PubMed] [Google Scholar]
- 34.Maltese W A, Erdman R A. J Biol Chem. 1989;264:18168–18172. [PubMed] [Google Scholar]
- 35.Davisson V J, Woodside A B, Neal T R, Stremler K E, Muehlbacher M, Poulter C D. J Org Chem. 1986;51:4768–4779. [Google Scholar]
- 36.Brown M J, Milano P D, Lever D C, Epstein W W, Poulter C D. J Am Chem Soc. 1991;113:3176–3177. [Google Scholar]
- 37.Reichlin M. Methods Enzymol. 1980;70:159–165. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z, Sun J, Pradines A, Favre G, Adnane J, Sebti S M. J Biol Chem. 2000;275:17974–17978. doi: 10.1074/jbc.C000145200. [DOI] [PubMed] [Google Scholar]
- 39.Rilling H C, Bruenger E, Leining L M, Buss J E, Epstein W W. Arch Biochem Biophys. 1993;301:210–215. doi: 10.1006/abbi.1993.1135. [DOI] [PubMed] [Google Scholar]
- 40.Khosravi-Far R, Der C J. In: Small GTPases and their Regulators, Part A. Balch W E, Der C J, Hall A, editors. Vol. 255. San Diego: Academic; 1995. pp. 46–60. [Google Scholar]