Abstract
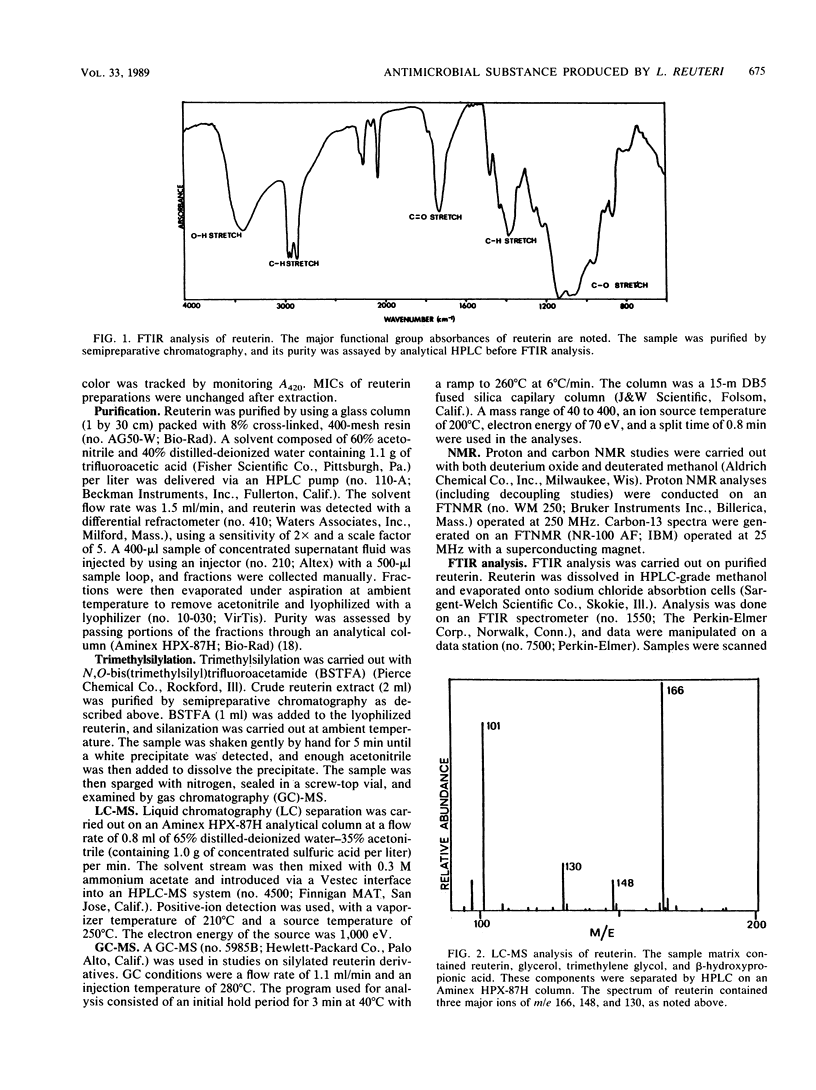
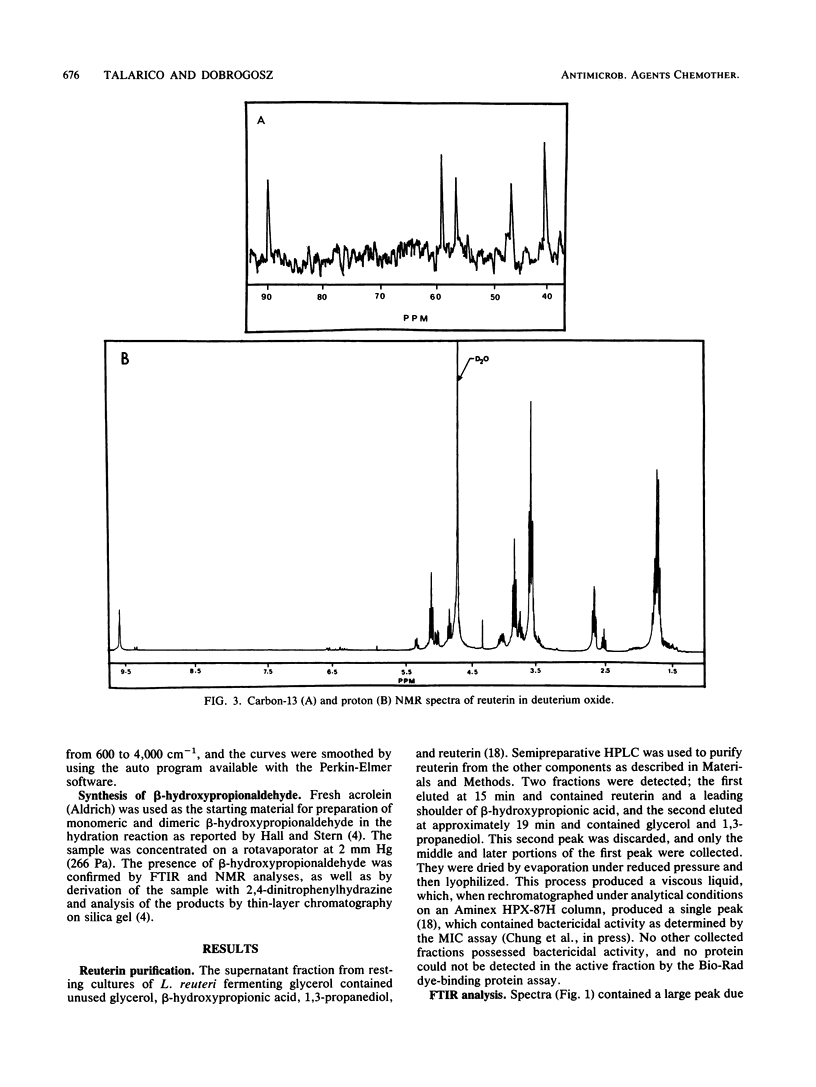
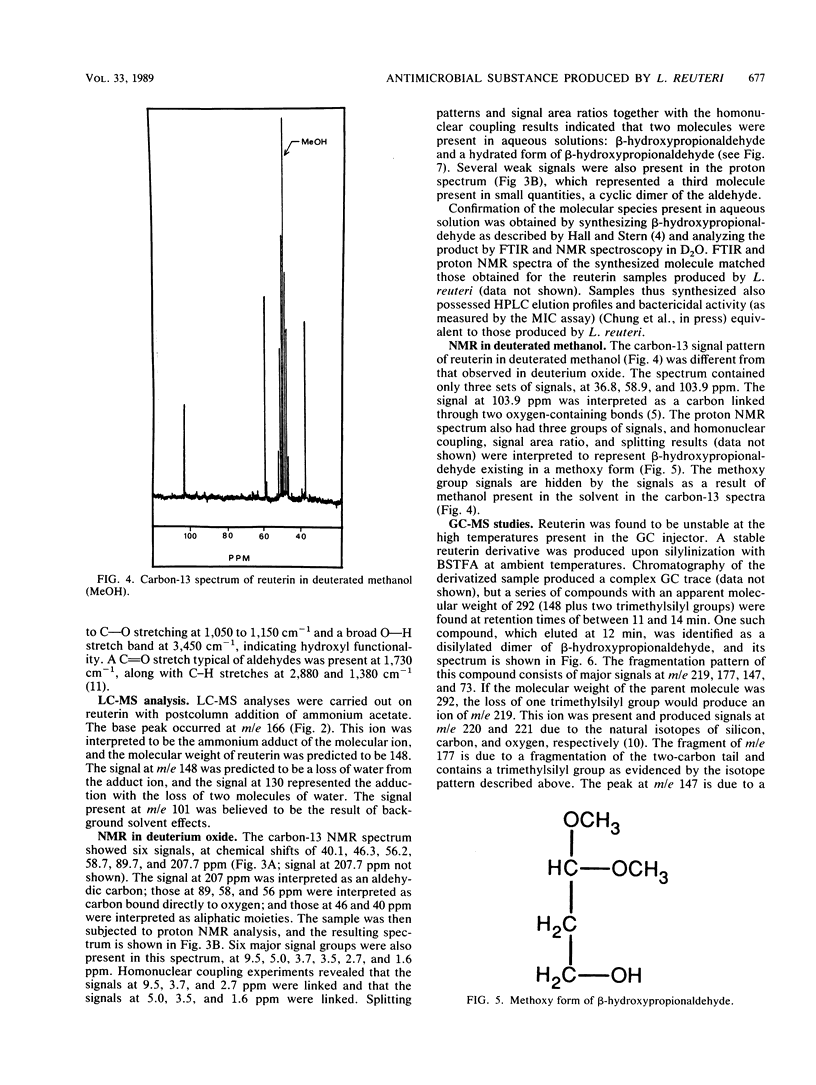
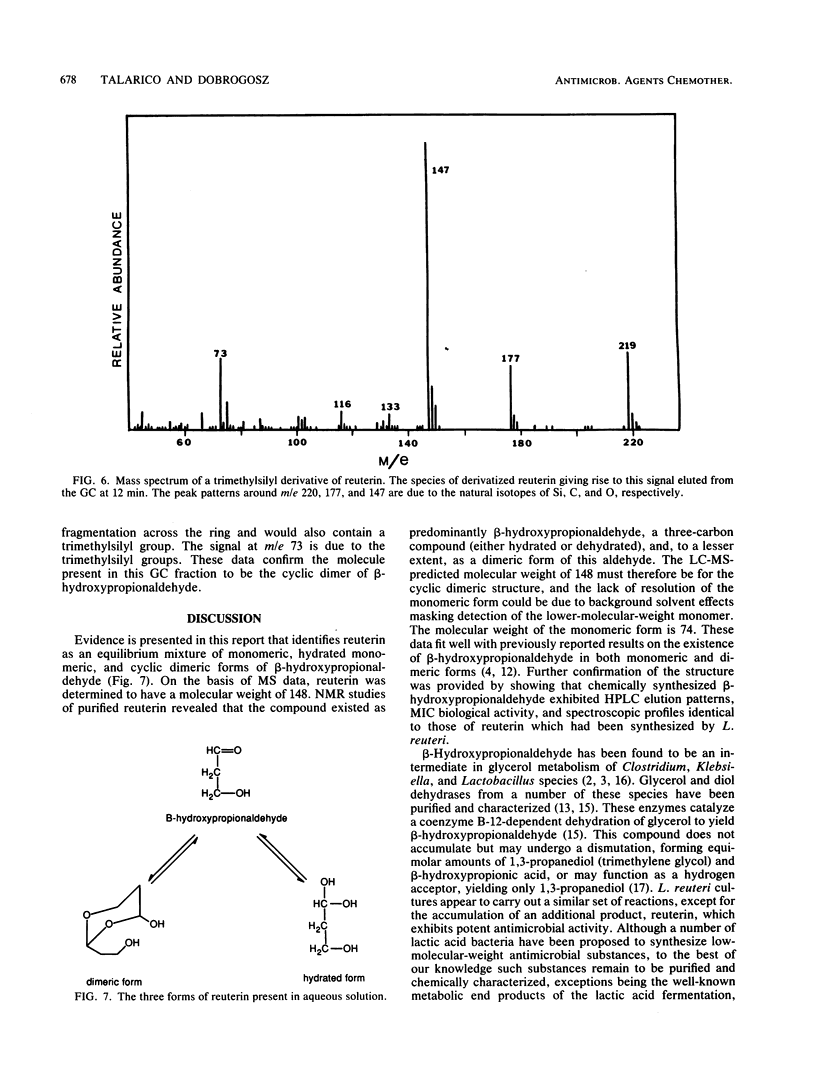
Lactobacillus reuteri converts glycerol into a potent cell growth inhibitor. This substance, termed reuterin, inhibits the growth of gram-positive and gram-negative bacteria as well as yeasts, fungi, and protozoa. Semipreparative chromatography was used to purify reuterin, and Fourier transform infrared spectroscopy and liquid chromatography-mass spectrometry were used to establish the molecular weight as well as the molecular functionality of the reuterin molecule. Nuclear magnetic resonance studies of purified reuterin carried out with deuterium oxide confirmed the presence of two three-carbon compounds, beta-hydroxypropionaldehyde and the corresponding hydrated acetal, and a six-carbon cyclic dimer of the aldehyde. Further nuclear magnetic resonance studies with deuterated methanol revealed that in this solvent the compound existed as a three-carbon compound in a methoxy form. Trimethylsilyl derivatives of reuterin were analyzed by gas chromatography-mass spectrometry, and a molecule was identified which had a molecular weight corresponding to a disilylated dimeric structure. On the basis of the above information, reuterin was determined to be an equilibrium mixture of monomeric, hydrated monomeric, and cyclic dimeric forms of beta-hydroxypropionaldehyde. This was subsequently confirmed by chemical synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson L., Lindgren S. Characterization and DNA homology of Lactobacillus strains isolated from pig intestine. J Appl Bacteriol. 1987 May;62(5):433–440. doi: 10.1111/j.1365-2672.1987.tb02673.x. [DOI] [PubMed] [Google Scholar]
- Forage R. G., Lin E. C. DHA system mediating aerobic and anaerobic dissimilation of glycerol in Klebsiella pneumoniae NCIB 418. J Bacteriol. 1982 Aug;151(2):591–599. doi: 10.1128/jb.151.2.591-599.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W. Production of 1,3-Propanediol from Glycerol by Clostridium acetobutylicum and Other Clostridium Species. Appl Environ Microbiol. 1987 Apr;53(4):639–643. doi: 10.1128/aem.53.4.639-643.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988 Mar;70(3):337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Poznanskaja A. A., Tanizawa K., Soda K., Toraya T., Fukui S. Coenzyme B12-dependent diol dehydrase: purification, subunit heterogeneity, and reversible association. Arch Biochem Biophys. 1979 May;194(2):379–386. doi: 10.1016/0003-9861(79)90630-1. [DOI] [PubMed] [Google Scholar]
- SMILEY K. L., SOBOLOV M. A cobamide-requiring glycerol dehydrase from an acrolein-forming Lactobacillus. Arch Biochem Biophys. 1962 Jun;97:538–543. doi: 10.1016/0003-9861(62)90118-2. [DOI] [PubMed] [Google Scholar]
- SOBOLOV M., SMILEY K. L. Metabolism of glycerol by an acrolein-forming lactobacillus. J Bacteriol. 1960 Feb;79:261–266. doi: 10.1128/jb.79.2.261-266.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider Z., Larsen E. G., Jacobson G., Johnson B. C., Pawelkiewicz J. Purification and properties of glycerol dehydrase. J Biol Chem. 1970 Jul 10;245(13):3388–3396. [PubMed] [Google Scholar]
- Talarico T. L., Casas I. A., Chung T. C., Dobrogosz W. J. Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob Agents Chemother. 1988 Dec;32(12):1854–1858. doi: 10.1128/aac.32.12.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Archibald R. D. The derivation and use of mice which do not harbour lactobacilli in the gastrointestinal tract. Can J Microbiol. 1984 Jun;30(6):849–853. doi: 10.1139/m84-131. [DOI] [PubMed] [Google Scholar]


