Abstract
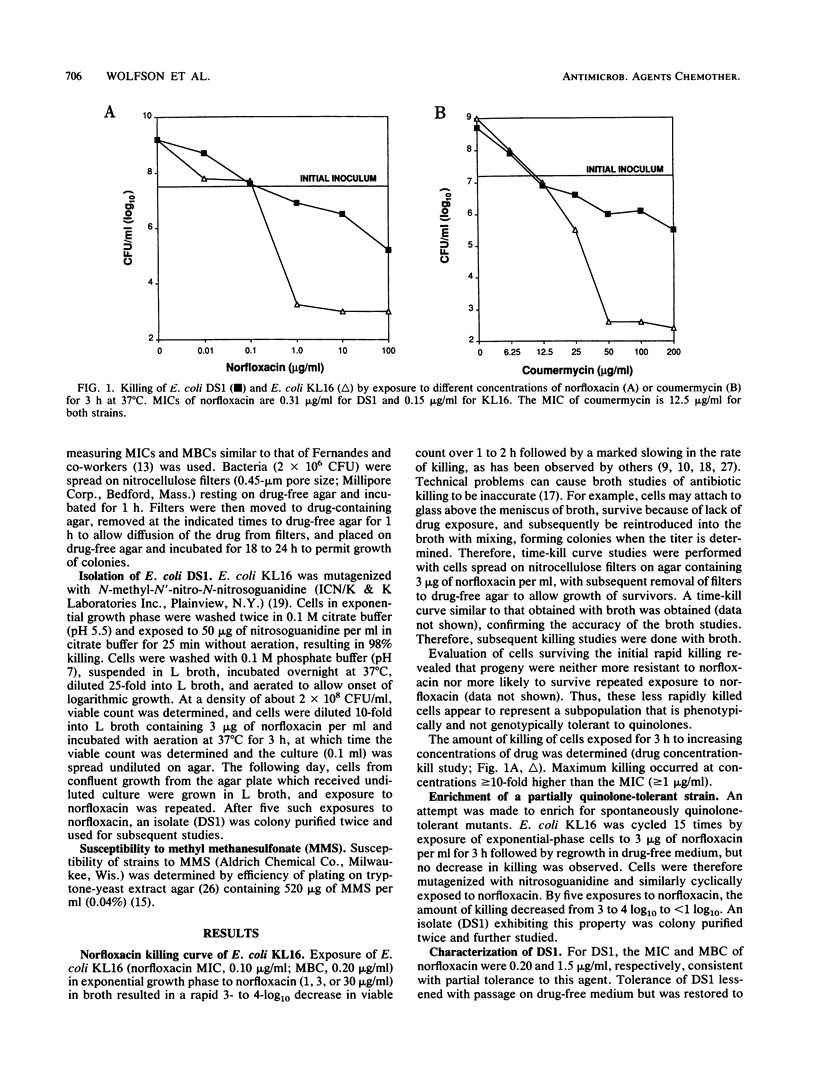
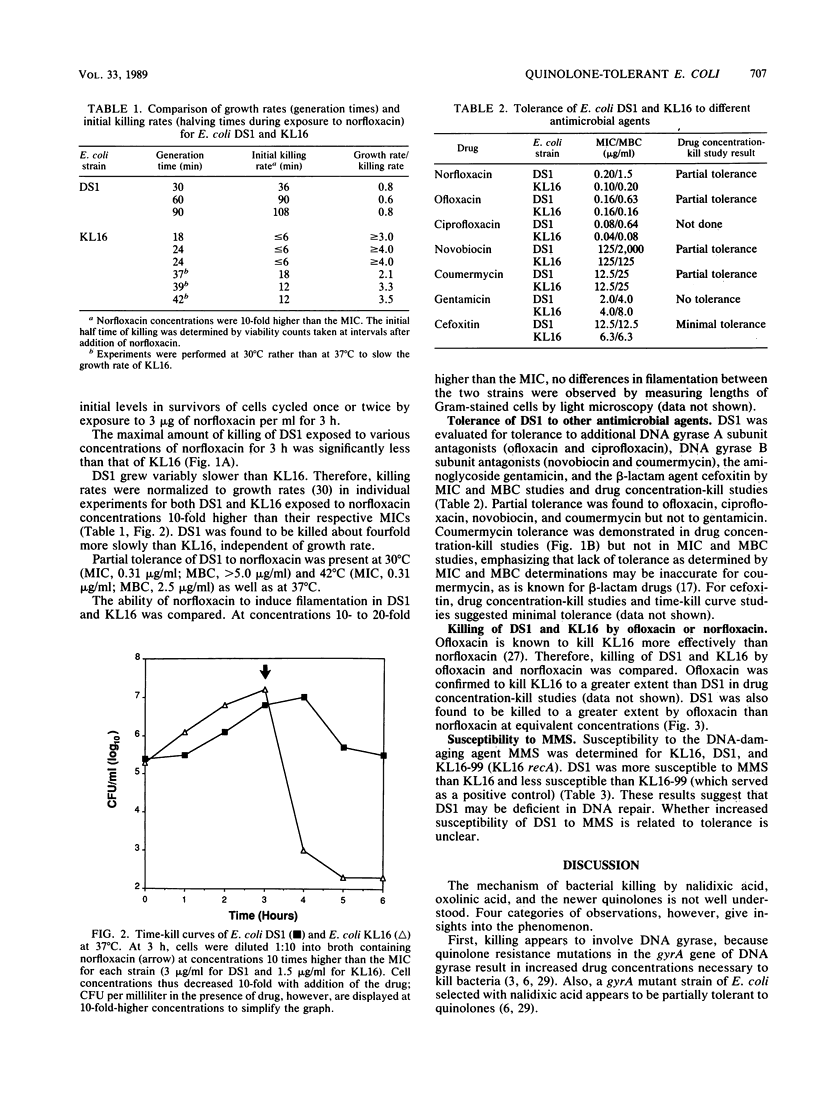
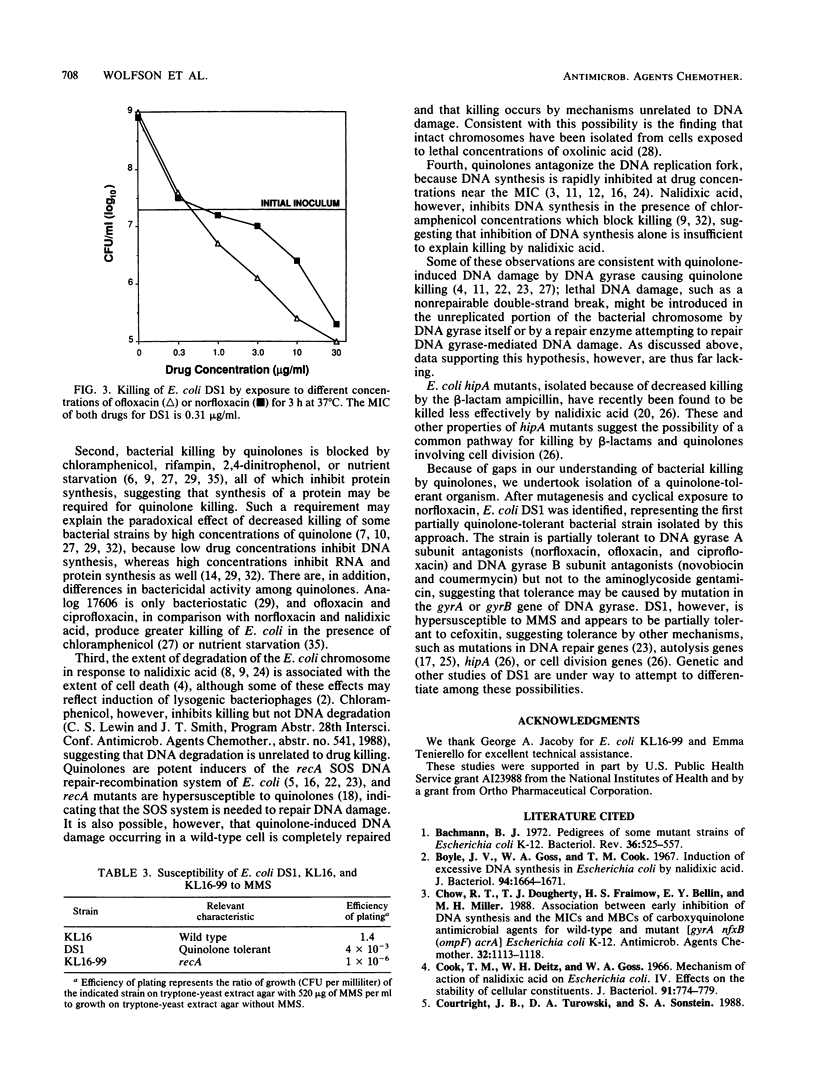
Quinolone antimicrobial agents rapidly kill bacteria by largely unknown mechanisms. To study this phenomenon, a strain of Escherichia coli inhibited but inefficiently killed by (i.e., partially tolerant to) norfloxacin was isolated and characterized. E. coli KL16 (norfloxacin MIC, 0.10 microgram/ml; MBC, 0.20 microgram/ml) was mutagenized with nitrosoguanidine and cyclically exposed to 3 micrograms of norfloxacin per ml. After five cycles, a bacterial strain (DS1) which was killed 1,000-fold less than KL16 during 3 h of drug exposure was isolated. The MIC and MBC of norfloxacin for DS1 were 0.20 and 1.5 micrograms/ml, respectively. Over a range of norfloxacin concentrations, DS1 was killed 2 to 4 orders of magnitude less than KL16. DS1 grew more slowly than KL16 but after normalization for growth rate was killed four times less rapidly than KL16 at drug concentrations 10-fold higher than respective MICs. DS1 and KL16 cells filamented similarly upon exposure to norfloxacin. DS1 exhibited tolerance to other DNA gyrase A subunit antagonists (ofloxacin and ciprofloxacin) and DNA gyrase B subunit antagonists (novobiocin and coumermycin) but not to the aminoglycoside gentamicin, suggesting involvement of DNA gyrase. DS1 also appeared to be minimally tolerant to the beta-lactam cefoxitin. DS1 exhibited increased susceptibility to the mutagen methyl methanesulfonate, implying a defect in DNA repair. This report describes the first use of quinolone enrichment for isolation of a bacterial strain partially tolerant to quinolones. The study of defects in such tolerant strains offers an approach to an increased understanding of the mechanisms of bacterial killing by quinolones.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. V., Goss W. A., Cook T. M. Induction of excessive deoxyribonucleic acid synthesis in Escherichia coli by nalidixic acid. J Bacteriol. 1967 Nov;94(5):1664–1671. doi: 10.1128/jb.94.5.1664-1671.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow R. T., Dougherty T. J., Fraimow H. S., Bellin E. Y., Miller M. H. Association between early inhibition of DNA synthesis and the MICs and MBCs of carboxyquinolone antimicrobial agents for wild-type and mutant [gyrA nfxB(ompF) acrA] Escherichia coli K-12. Antimicrob Agents Chemother. 1988 Aug;32(8):1113–1118. doi: 10.1128/aac.32.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook T. M., Deitz W. H., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. IV. Effects on the stability of cellular constituents. J Bacteriol. 1966 Feb;91(2):774–779. doi: 10.1128/jb.91.2.774-779.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtright J. B., Turowski D. A., Sonstein S. A. Alteration of bacterial DNA structure, gene expression, and plasmid encoded antibiotic resistance following exposure to enoxacin. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):1–18. doi: 10.1093/jac/21.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- Crumplin G. C., Kenwright M., Hirst T. Investigations into the mechanism of action of the antibacterial agent norfloxacin. J Antimicrob Chemother. 1984 May;13 (Suppl B):9–23. doi: 10.1093/jac/13.suppl_b.9. [DOI] [PubMed] [Google Scholar]
- Crumplin G. C., Smith J. T. Nalidixic acid and bacterial chromosome replication. Nature. 1976 Apr 15;260(5552):643–645. doi: 10.1038/260643a0. [DOI] [PubMed] [Google Scholar]
- Crumplin G. C., Smith J. T. Nalidixic acid: an antibacterial paradox. Antimicrob Agents Chemother. 1975 Sep;8(3):251–261. doi: 10.1128/aac.8.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitz W. H., Cook T. M., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. 3. Conditions required for lethality. J Bacteriol. 1966 Feb;91(2):768–773. doi: 10.1128/jb.91.2.768-773.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diver J. M., Wise R. Morphological and biochemical changes in Escherichia coli after exposure to ciprofloxacin. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):31–41. doi: 10.1093/jac/18.supplement_d.31. [DOI] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Franco R. J. Inhibitors of DNA topoisomerases. Biochemistry. 1988 Apr 5;27(7):2253–2259. doi: 10.1021/bi00407a001. [DOI] [PubMed] [Google Scholar]
- Fernandes C. J., Stevens D. A., Groot obbink D. J., Ackerman V. P. A replicator method for the combined determination of minimum inhibitory concentration and minimum bactericidal concentration. J Antimicrob Chemother. 1985 Jan;15(1):53–60. doi: 10.1093/jac/15.1.53. [DOI] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Zipser D. Deg phenotype of Escherichia coli lon mutants. J Bacteriol. 1978 Feb;133(2):844–851. doi: 10.1128/jb.133.2.844-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Tomasz A. Antibiotic tolerance among clinical isolates of bacteria. Rev Infect Dis. 1985 May-Jun;7(3):368–386. doi: 10.1093/clinids/7.3.368. [DOI] [PubMed] [Google Scholar]
- McDaniel L. S., Rogers L. H., Hill W. E. Survival of recombination-deficient mutants of Escherichia coli during incubation with nalidixic acid. J Bacteriol. 1978 Jun;134(3):1195–1198. doi: 10.1128/jb.134.3.1195-1198.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyed H. S., Bertrand K. P. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol. 1983 Aug;155(2):768–775. doi: 10.1128/jb.155.2.768-775.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. Clinical use of the quinolones. Lancet. 1987 Dec 5;2(8571):1319–1322. doi: 10.1016/s0140-6736(87)91205-0. [DOI] [PubMed] [Google Scholar]
- Phillips I., Culebras E., Moreno F., Baquero F. Induction of the SOS response by new 4-quinolones. J Antimicrob Chemother. 1987 Nov;20(5):631–638. doi: 10.1093/jac/20.5.631. [DOI] [PubMed] [Google Scholar]
- Ramareddy G., Reiter H. Specific loss of newly replicated deoxyribonucleic acid in nalidixic acid-treated Bacillus subtilis 168. J Bacteriol. 1969 Nov;100(2):724–729. doi: 10.1128/jb.100.2.724-729.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronda C., García J. L., García E., Sánchez-Puelles J. M., López R. Biological role of the pneumococcal amidase. Cloning of the lytA gene in Streptococcus pneumoniae. Eur J Biochem. 1987 May 4;164(3):621–624. doi: 10.1111/j.1432-1033.1987.tb11172.x. [DOI] [PubMed] [Google Scholar]
- Scherrer R., Moyed H. S. Conditional impairment of cell division and altered lethality in hipA mutants of Escherichia coli K-12. J Bacteriol. 1988 Aug;170(8):3321–3326. doi: 10.1128/jb.170.8.3321-3326.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Drlica K. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J Mol Biol. 1979 Jun 25;131(2):287–302. doi: 10.1016/0022-2836(79)90077-9. [DOI] [PubMed] [Google Scholar]
- Stevens P. J. Bactericidal effect against Escherichia coli of nalidixic acid and four structurally related compounds. J Antimicrob Chemother. 1980 Jul;6(4):535–542. doi: 10.1093/jac/6.4.535. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Cozens R., Tosch W., Zak O., Tomasz A. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol. 1986 May;132(5):1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Recent studies of DNA topoisomerases. Biochim Biophys Acta. 1987 Jun 6;909(1):1–9. doi: 10.1016/0167-4781(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Winshell E. B., Rosenkranz H. S. Nalidixic Acid and the Metabolism of Escherichia coli. J Bacteriol. 1970 Dec;104(3):1168–1175. doi: 10.1128/jb.104.3.1168-1175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler H. J. Evaluation of the in vitro bactericidal action of ciprofloxacin on cells of Escherichia coli in the logarithmic and stationary phases of growth. Antimicrob Agents Chemother. 1985 Oct;28(4):524–527. doi: 10.1128/aac.28.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]


