Abstract
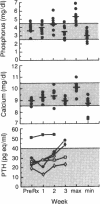
Ten patients with acquired immunodeficiency syndrome with newly diagnosed cytomegalovirus (CMV) retinitis were treated with an induction regimen of intravenous foscarnet, 60 mg/kg of body weight, administered as a 2-h infusion and repeated every 8 h for 14 days. At the end of induction, 9 of 10 patients had stabilized (no new retinal lesions and stable old lesions [7 patients]) or improved (decreased retinal opacification [2 patients]). All eight patients with CMV in urine or blood upon entry into the study had negative urine and blood cultures at the end of induction. After induction therapy, seven patients continued maintenance foscarnet therapy, 60 mg/kg as a single daily infusion, 5 days/week. In six patients, retinal lesions increased in size after 2 to 32 weeks of maintenance therapy. One was invaluable because a retinal detachment developed. Only 9 of 42 blood and urine cultures obtained during maintenance foscarnet therapy yielded CMV, compared with 7 of 14 obtained prior to the initiation of foscarnet induction therapy (P = 0.04). Foscarnet toxicity was mild and infrequent: elevation in serum creatinine by 0.5 to 1.3 mg/dl over the base line (two patients), muscle twitching (three patients), hemoglobin decrease by 1 mg/dl (two patients), nausea (two patients), absolute neutrophil count decrease by 50% (one patient), rise in serum phosphorus to greater than 5.5 mg/dl (four patients), and proteinuria (two patients). Intermittently administered intravenous foscarnet appears to be an effective, relatively nontoxic therapy for CMV retinitis. Additional studies to determine the optimal dosage for maintenance therapy are needed, as are comparative trials with ganciclovir.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akesson-Johansson A., Lernestedt J. O., Ringdén O., Lönnqvist B., Wahren B. Sensitivity of cytomegalovirus to intravenous foscarnet treatment. Bone Marrow Transplant. 1986 Dec;1(2):215–220. [PubMed] [Google Scholar]
- Buhles W. C., Jr, Mastre B. J., Tinker A. J., Strand V., Koretz S. H. Ganciclovir treatment of life- or sight-threatening cytomegalovirus infection: experience in 314 immunocompromised patients. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 3):S495–S506. doi: 10.1093/clinids/10.supplement_3.s495. [DOI] [PubMed] [Google Scholar]
- Holland G. N., Sakamoto M. J., Hardy D., Sidikaro Y., Kreiger A. E., Frenkel L. M. Treatment of cytomegalovirus retinopathy in patients with acquired immunodeficiency syndrome. Use of the experimental drug 9-[2-hydroxy-1-(hydroxymethyl)ethoxymethyl]guanine. Arch Ophthalmol. 1986 Dec;104(12):1794–1800. doi: 10.1001/archopht.1986.01050240068042. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A., O'Donnell J. J., Brodie H. R., Wofsy C., Mills J. Randomized prospective trial of ganciclovir maintenance therapy for cytomegalovirus retinitis. J Med Virol. 1988 Jul;25(3):339–349. doi: 10.1002/jmv.1890250311. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A., O'Donnell J. J., Porteous D., Brodie H. R., Feigal D., Mills J. Retinal and gastrointestinal disease due to cytomegalovirus in patients with the acquired immune deficiency syndrome: prevalence, natural history, and response to ganciclovir therapy. Q J Med. 1988 Jun;67(254):473–486. [PubMed] [Google Scholar]
- Klintmalm G., Lönnqvist B., Oberg B., Gahrton G., Lernestedt J. O., Lundgren G., Ringdén O., Robert K. H., Wahren B., Groth C. G. Intravenous foscarnet for the treatment of severe cytomegalovirus infection in allograft recipients. Scand J Infect Dis. 1985;17(2):157–163. doi: 10.3109/inf.1985.17.issue-2.06. [DOI] [PubMed] [Google Scholar]
- Laskin O. L., Stahl-Bayliss C. M., Kalman C. M., Rosecan L. R. Use of ganciclovir to treat serious cytomegalovirus infections in patients with AIDS. J Infect Dis. 1987 Feb;155(2):323–327. doi: 10.1093/infdis/155.2.323. [DOI] [PubMed] [Google Scholar]
- Mar E. C., Cheng Y. C., Huang E. S. Effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine on human cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 1983 Oct;24(4):518–521. doi: 10.1128/aac.24.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringdén O., Lönnqvist B., Paulin T., Ahlmén J., Klintmalm G., Wahren B., Lernestedt J. O. Pharmacokinetics, safety and preliminary clinical experiences using foscarnet in the treatment of cytomegalovirus infections in bone marrow and renal transplant recipients. J Antimicrob Chemother. 1986 Mar;17(3):373–387. doi: 10.1093/jac/17.3.373. [DOI] [PubMed] [Google Scholar]
- Ringdén O., Wilczek H., Lönnqvist B., Gahrton G., Wahren B., Lernestedt J. O. Foscarnet for cytomegalovirus infections. Lancet. 1985 Jun 29;1(8444):1503–1504. doi: 10.1016/s0140-6736(85)92272-x. [DOI] [PubMed] [Google Scholar]
- Sandstrom E. G., Kaplan J. C., Byington R. E., Hirsch M. S. Inhibition of human T-cell lymphotropic virus type III in vitro by phosphonoformate. Lancet. 1985 Jun 29;1(8444):1480–1482. doi: 10.1016/s0140-6736(85)92255-x. [DOI] [PubMed] [Google Scholar]
- Tocci M. J., Livelli T. J., Perry H. C., Crumpacker C. S., Field A. K. Effects of the nucleoside analog 2'-nor-2'-deoxyguanosine on human cytomegalovirus replication. Antimicrob Agents Chemother. 1984 Feb;25(2):247–252. doi: 10.1128/aac.25.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley S. L., Chew E., Read S. E., Vellend H., Salit I., Rachlis A., Fanning M. M. Treatment of cytomegalovirus retinitis with trisodium phosphonoformate hexahydrate (Foscarnet). J Infect Dis. 1988 Mar;157(3):569–572. doi: 10.1093/infdis/157.3.569. [DOI] [PubMed] [Google Scholar]
- Yusufi A. N., Szczepanska-Konkel M., Kempson S. A., McAteer J. A., Dousa T. P. Inhibition of human renal epithelial Na+/Pi cotransport by phosphonoformic acid. Biochem Biophys Res Commun. 1986 Sep 14;139(2):679–686. doi: 10.1016/s0006-291x(86)80044-4. [DOI] [PubMed] [Google Scholar]