Abstract
Light induces phosphorylation of photosystem II (PSII) proteins in chloroplasts by activating the protein kinase(s) via reduction of plastoquinone and the cytochrome b6f complex. The recent finding of high-light-induced inactivation of the phosphorylation of chlorophyll a/b-binding proteins (LHCII) of the PSII antenna in floated leaf discs, but not in vitro, disclosed a second regulatory mechanism for LHCII phosphorylation. Here we show that this regulation of LHCII phosphorylation is likely to be mediated by the chloroplast ferredoxin–thioredoxin system. We present a cooperative model for the function of the two regulation mechanisms that determine the phosphorylation level of the LHCII proteins in vivo, based on the following results: (i) Chloroplast thioredoxins f and m efficiently inhibit LHCII phosphorylation. (ii) A disulfide bond in the LHCII kinase, rather than in its substrate, may be a target component regulated by thioredoxin. (iii) The target disulfide bond in inactive LHCII kinase from dark-adapted leaves is exposed and easily reduced by external thiol mediators, whereas in the activated LHCII kinase the regulatory disulfide bond is hidden. This finding suggests that the activation of the kinase induces a conformational change in the enzyme. The active state of LHCII kinase prevails in chloroplasts under low-light conditions, inducing maximal phosphorylation of LHCII proteins in vivo. (iv) Upon high-light illumination of leaves, the target disulfide bond becomes exposed and thus is made available for reduction by thioredoxin, resulting in a stable inactivation of LHCII kinase.
Reversible protein phosphorylation is a unique regulatory mechanism for modification of the structure and function of proteins in both prokaryotic and eukaryotic organisms. It plays a fundamental role in signal transduction pathways that relay information from outside of the cell to the gene level. Bennett (1, 2) discovered the reversible, light-dependent phosphorylation of proteins in thylakoid membranes of plant chloroplasts. All thylakoid phosphoproteins identified so far are closely associated with photosystem II (PSII), a light-driven water—plastoquinone–oxidoreductase enzyme. Four core proteins of PSII, including the D1 and D2 reaction center proteins, the 43-kDa chlorophyll a-binding protein (CP43 protein), and the psbH gene product (PsbH protein) are prone to redox-regulated reversible phosphorylation. Three of six chlorophyll a/b-binding proteins of the PSII antenna, Lhcb1 and Lhcb2 (designated LHCII) as well as Lhcb4 proteins, also undergo light-dependent phosphorylation. The protein kinase(s) involved in phosphorylation of the PSII proteins is associated with thylakoid membranes (2) and is activated by light via reduction of electron transfer components, plastoquinone and cytochrome b6/f complex (2–6).
A large majority of the experimental data support the existence of two different kinases for PSII phosphoproteins, one for LHCII and another for PSII core proteins with distinct redox regulation systems (3, 6–10). The PSII core protein kinase is activated via reduction of plastoquinone (3, 6), whereas the LHCII kinase appears to interact directly with the cytochrome b6f complex (6, 11). It has recently been shown that the LHCII kinase remains active as long as a plastoquinol molecule is bound to the Qo site of the cytochrome b6f complex (5).
We have recently shown that the maximal phosphorylation of LHCII polypeptides in vivo only occurs at low light intensities. A strong down-regulation of LHCII phosphorylation takes place at higher irradiances (9, 12). Interestingly, such an inactivation of LHCII protein phosphorylation did not occur under illumination of isolated thylakoids as such, but could be rapidly induced by the addition of thiol-reducing compounds (9, 10). This observation suggests that besides the cytochrome b6f complex-dependent activation of the LHCII kinase, phosphorylation of LHCII polypeptides in vivo is further regulated by a mechanism not present in the thylakoid membrane. In this paper a regulation model for LHCII phosphorylation, in vivo under varying environmental conditions, is presented. Such a regulation involves a coordinated and specific interaction of the two mechanisms, the activation of LHCII kinase by reduction of the cytochrome b6f complex and the inhibition of LHCII phosphorylation by thiol reagents, which are both strongly dependent on environmental conditions. We also provide evidence that chloroplast thioredoxins mediate the thiol-based inhibition of LHCII kinase in vivo.
Materials and Methods
Plant Material.
Pumpkin (Cucurbita pepo L.) plants were grown in a greenhouse at 20°C with a 16-h photoperiod at a photon flux density of 200 μmol·m−2·s−1. Fully expanded 4- to 5-week-old leaves were used in the experiments.
Light Treatment of the Leaf Discs.
Leaf discs (diameter 2.7 cm), punched from dark-adapted leaves and floating on distilled water in a Petri dish, were illuminated in a growth chamber at 23°C under irradiances indicated in the figures. A metal halide lamp (HQI-T, 250 W for daylight) served as the light source. Leaf discs, instead of intact leaves, were illuminated to obtain uniformly phosphorylated thylakoid membranes (9). For direct analysis of the thylakoid phosphoproteins in vivo, the leaf discs were rapidly frozen in liquid nitrogen and stored at −80°C. Thylakoid membranes were isolated as described previously (13).
In Vitro Phosphorylation Assay of Thylakoid Membranes Isolated from Differentially Illuminated Leaves.
Thylakoid membranes isolated from dark-adapted leaves or plants illuminated for 1 h at a photon flux density of 50 μmol·m−2·s−1 or 1000 μmol⋅m−2⋅s−1 were resuspended in assay buffer consisting of 50 mM Hepes-NaOH (pH 7.5), 100 mM sucrose, 5 mM NaCl, 10 MgCl2, and 10 mM NaF at a final chlorophyll concentration of 0.4 mg·ml−1. Before phosphorylation assays, isolated thylakoid membranes were preincubated at 23°C for 5–10 min either in darkness or in light, in the presence of the following chemicals: l-ascorbic acid (Sigma); 3-(3,4-dichlorophenyl)-1,1-dimethylurea (Sigma); 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (TCI America, Portland, OR); DTT (Roche Molecular Biochemicals); trans-4,5-dihydroxy-1,2-dithiane (Sigma); reduced and oxidized glutathione (Sigma); N-ethylmaleimide (NEM) (Sigma); or chloroplast thioredoxin f and m of pea. Thioredoxins f and m were kept reduced by 0.2 mM DTT in the assay medium. The concentrations of the chemicals are indicated in the figures and in the table. Thylakoid protein phosphorylation was initiated by the addition of 0.4 mM ATP under a photon flux density of 100 μmol·m−2·s−1. Phosphorylation of thylakoid proteins in darkness was assayed in the presence of 1 mM NADPH (Roche Molecular Biochemicals) and 10 μM ferredoxin (Sigma) as described in (14).
Detection of Thylakoid Phosphoproteins by Polyclonal Phosphothreonine Antibody.
Thylakoids were solubilized in the presence of 6 M urea, and the polypeptides were separated by SDS-PAGE (15), using 15% (wt/vol) acrylamide gels with 6 M urea. Routinely, 1.0 μg of chlorophyll was loaded into each well. The polypeptides were transferred to an Immobilon-P membrane (Millipore), and the membrane was blocked with 1% BSA (fatty-acid free; Sigma). Phosphoproteins were immunodetected using a Biolabs Phototope-Star Chemiluminescent Kit (New England Biolabs) with rabbit polyclonal phosphothreonine antibody (Zymed) as described in (9). For quantification of phosphoproteins, the immunoblots were scanned using an image program (Imaging Research, St. Catharine's, ON, Canada). The phosphorylation level of the two light-harvesting proteins Lhcb1 and Lhcb2 (designed LHCII) and that of the D1 protein (representing PSII core phosphoproteins) is presented in the figures. It should be noted that the analytical method using phosphothreonine antibody does not allow a direct comparison of the phosphorylation levels of different phosphoproteins, but efficiently describes the changes occurring in the phosphorylation of each phosphoprotein (9).
Chlorophyll Determinations.
Chlorophyll was extracted in 80% buffered acetone and determined as described previously (16).
Results
Differential Phosphorylation of D1 and LHCII Proteins Under Various Redox Conditions in Vitro and in Vivo.
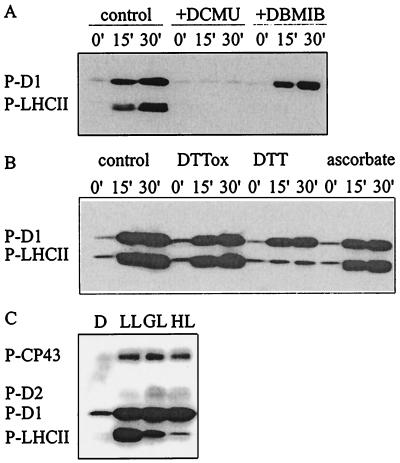
In vitro phosphorylation of thylakoid membranes, representing the control for most experiments in this study, resulted in similar phosphorylation levels of D1 and LHCII proteins observed in vivo under optimal phosphorylation conditions (Fig. 1). These levels correspond to about 80% and 20% of all D1 and LHCII proteins in thylakoids, respectively (9, 17). Application of photosynthetic electron transfer inhibitors in vitro indicated that phosphorylation of D1 and LHCII proteins is regulated differentially by the reduction state of thylakoid redox components (Fig. 1A). 3-(3,4-Dichlorophenyl)-1,1-dimethylurea inhibited phosphorylation of all PSII proteins by blocking the electron transfer between PSII and the plastoquinone pool. 2,5-Dibromo-3-methyl-6-isopropyl-p-benzoquinone, blocking the reduction of cytochrome b6f complex, prevented LHCII phosphorylation without any effect on D1 phosphorylation (Fig.1A). Thiol-reducing agents had a similar dual effect on PSII protein phosphorylation (Fig. 1B) (10). Moreover, PSII and LHCII protein phosphorylation was differentially regulated by ambient light conditions in vivo (Fig. 1C); at high irradiances, a strong down-regulation of LHCII phosphorylation, but not that of PSII core proteins, was observed. The data presented in Fig. 1 strongly suggest that two kinases are involved in PSII protein phosphorylation, with distinct redox regulation via thylakoid redox components and the thiol redox state of chloroplasts.
Figure 1.
Modulation of PSII protein phosphorylation by electron transfer inhibitors, reducing agents, and quantity of light. (A and B) Thylakoids isolated from dark-adapted leaves were phosphorylated in vitro for 15 or 30 min in the presence of the following reagents: 20 μM 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) or 10 μM 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB) (A) or 2 mM trans-4,5-dihydroxy-1,2-dithiane (DTTox), 2 mM DTT, or 10 mM ascorbate (B). Immunoblots present the phosphorylation level of the D1 and LHCII proteins. (C) Steady-state phosphorylation level of PSII proteins in vivo. Leaf discs were incubated in darkness (D) or illuminated for 2 h at a photon flux density of 30 (LL), 200 (GL), or 1000 (HL) μmol·m−2·s−1. Phosphorylated PSII proteins were detected by immunoblotting with phosphothreonine antibody. P-CP43, P-D2, P-D1, and P-LHCII, phosphorylated forms of CP43, D2, D1, and LHCII proteins, respectively.
Effect of Natural and Artificial Thiol-Modifying Agents on PSII Protein Phosphorylation in Thylakoid Membranes Isolated from Dark-Adapted Leaves.
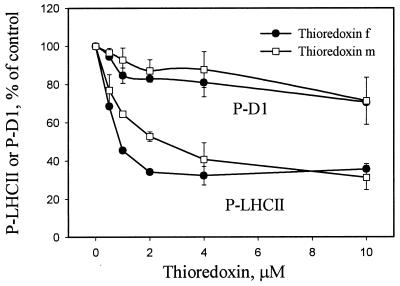
We first studied the effects of physiological thiol mediators in chloroplast on the phosphorylation of PSII proteins. As shown in Fig. 2, both thioredoxins f and m inactivated the phosphorylation of LHCII proteins without any significant effect on D1 phosphorylation, thioredoxin f being more efficient at low concentrations (1–4 μM) than thioredoxin m. This difference was abolished at higher concentrations of the mediator, when a slight inhibition of D1 protein phosphorylation was also detected (Fig. 2).
Figure 2.
Effect of chloroplast thioredoxins on the phosphorylation of LHCII and D1 proteins. Thylakoids isolated from dark-adapted leaves were incubated in darkness in the presence of thioredoxin f or m at the concentrations indicated. DTT (0.2 mM) was included in the incubation medium to keep thioredoxins in their reduced form. A minor effect of DTT on thylakoid protein phosphorylation (see Fig. 3) was always subtracted from the inhibition observed with thioredoxins. After incubation for 10 min in darkness, protein phosphorylation was initiated by the addition of ATP and turning the light on. Phosphorylation levels of proteins were detected as described in Fig. 1, and the blots were quantified by scanning. Results are means ± SD, n = 2.
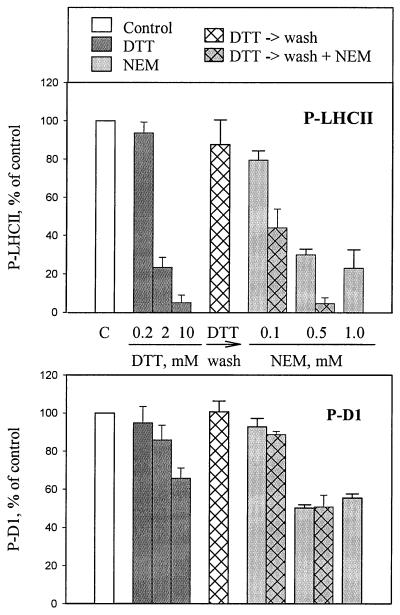
To obtain further information on the regulatory system mediated via the thiol redox state, we studied more closely the effects of reagents that either reduce the disulfide bridges in proteins (DTT and glutathione) or block the thiols by binding irreversibly to the SH groups of proteins (NEM). Specific inhibition of LHCII phosphorylation was observed at 2–5 mM concentrations of DTT (Fig. 3) (10). Higher concentrations of DTT (5–20 mM) also had a slight effect on D1 protein phosphorylation. The DTT-induced specific inhibition of LHCII phosphorylation could be reverted by washing out the thiol reagent. Subsequent incubation for 10 min at room temperature resulted in full restoration of LHCII phosphorylation (Fig. 3).
Figure 3.
Inhibition of LHCII and D1 protein phosphorylation by thiol reagents. Thylakoids isolated from dark-adapted leaves were incubated in darkness without (white bar) and with DTT (dark gray bars) or NEM (light gray bars) for 10 min. Thereafter, in vitro protein phosphorylation was initiated. In experiments indicated by hatched bars, the thylakoid membranes were first incubated with 2 mM DTT, which was subsequently washed out by pelleting the thylakoids and resuspending them in the same buffer either without thiol reagents (hatched white bars) or with various concentrations of NEM (hatched gray bars), and incubated in darkness for 10 min before the phosphorylation assay. The concentrations of DTT and NEM are indicated under (or above) the bars. The phosphorylation levels of the D1 and LHCII proteins were analyzed as described in Fig. 2. Results are means ± SD, n = 2–4.
A chloroplast protein kinase involved in phosphorylation of the plastid RNA polymerase has been found to be regulated by glutathione (18). However, glutathione was not suitable for our assay system: both reduced and oxidized glutathione (5 mM) induced complete inhibition of thylakoid protein phosphorylation (data not shown). Thus, we cannot at present eliminate the possibility that in vivo glutathione may also be a redox regulator of LHCII phosphorylation.
Thiol-modifying reagent NEM at concentrations from 0.5 to 1 mM strongly reduced the phosphorylation of both LHCII and D1 proteins (Fig. 3), whereas at low concentration of 0.1 mM, NEM exerted only a slight effect on the phosphorylation of these proteins. On the contrary, significant inhibition of LHCII protein phosphorylation was obtained if, before the addition of 0.1 mM NEM, the disulfide bonds of thylakoid proteins were reduced by pretreatment with 2 mM DTT, which was subsequently removed from the medium (Fig. 3). Notably, this NEM-induced inhibition of phosphorylation after pretreatment with DTT was specific for LHCII, with no effect on D1 phosphorylation (Fig. 3). These experiments provide evidence that the target regulatory thiols involved in the specific inhibition of LHCII phosphorylation are oxidized in thylakoids isolated from dark-adapted leaves and thus are not accessible for reaction with NEM. In the presence of thioredoxins or DTT, however, this bond undergoes a disulfide–sulfhydryl exchange reaction and subsequently becomes prone to modification by NEM.
Interaction between Cytochrome b6f-Dependent Activation of LHCII Kinase and Thiol-Redox State-Induced Inactivation of LHCII Phosphorylation.
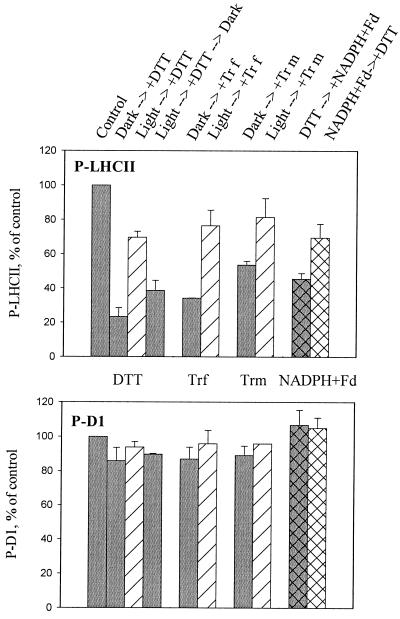
In dark-adapted thylakoid membranes used in the treatments described above, the LHCII kinase was inactive with respect to cytochrome b6f-dependent activation at the moment when the thiol mediators were introduced. To study the interaction of the two regulatory mechanisms of LHCII phosphorylation, activation via reduction of the cytochrome b6f complex and inactivation by thiol mediators, we next investigated thylakoids that had their LHCII kinase in an active form. Thus, the LHCII kinase was first activated by illumination of thylakoid membranes, and the thiol reductants were only introduced subsequently. After further illumination for 5 min, the phosphorylation of thylakoid proteins was initiated by the addition of ATP (Fig. 4). The phosphorylation of LHCII proteins was significantly less sensitive to thiol mediators in such thylakoids than in dark-adapted thylakoids with inactive LHCII kinase (Fig. 4). This reduced sensitivity was also observed in the presence of nigericin (data not shown), indicating that the light-induced transmembrane proton gradient was not involved in the process. To exclude the possibility that preillumination changed the effective concentration of DTT and thereby exerted its effect on LHCII phosphorylation, thylakoid assay medium containing DTT was first illuminated and then transferred to darkness for 10 min before the phosphorylation assay (light → +DTT → dark treatment in Fig. 4). The ability of DTT to inhibit LHCII phosphorylation was restored in darkness before the phosphorylation assay (Fig. 4), indicating that the reduced inhibition of LHCII phosphorylation in preilluminated thylakoids depends specifically on the modulation of the LHCII phosphorylation system, rather than on DTT concentration.
Figure 4.
Activation of LHCII kinase decreases the sensitivity of LHCII phosphorylation to thiol reagents. Thylakoids isolated from dark-adapted leaves were incubated for 5 min either in darkness (gray bars) or in light (striped bars). Thiol reagents were subsequently added, and incubation continued for 5 more min before initiation of the phosphorylation assay. Activation of the kinase and phosphorylation of thylakoid proteins were also conducted in total darkness in the presence of NADPH and ferredoxin (hatched bars). The sequential order of the addition of thiol reagents and NADPH + ferredoxin is indicated in the figure. In all cases, the thylakoid protein phosphorylation was initiated by the addition of ATP. Phosphorylation levels of LHCII and D1 proteins were identical in the control assays without thiol reagents, regardless of the activation of the kinase in darkness or in light before the initiation of phosphorylation. DTT (2 mM) and thioredoxins (2 μM) were used in the assay medium. The phosphorylation levels of the D1 and LHCII proteins were analyzed as described in Fig. 2. Results are means ± SD, n = 2–4.
We next asked whether the LHCII proteins as a substrate or the kinase phosphorylating these proteins is a target component that is regulated by the thiol redox state. The substrate, LHCII protein, is a conceivable candidate, as it has recently been reported that excitation of chlorophylls bound to LHCII apoproteins induces conformational changes in the LHCII, resulting in stimulation of its phosphorylation (19). The involvement of such light-induced mechanisms in the thiol regulation of LHCII phosphorylation was tested by activating the LHCII kinase in darkness by the addition of NADPH and ferredoxin (14). The presence of DTT in the assay medium, before activation of the kinase in darkness, resulted in 55% inhibition of LHCII phosphorylation capacity (Fig. 4). The opposite order, activation of the LHCII kinase first in darkness before the addition of DTT, induced significantly less inhibition of LHCII phosphorylation, only 25% (Fig. 4). Indeed, light as such was not required to attenuate the thiol mediator-dependent inhibition of LHCII phosphorylation; the crucial point was the activation state of the kinase. Thus, the likely target for thiol redox regulation of LHCII phosphorylation is a specific disulfide–sulfhydryl exchange in the enzyme, the LHCII kinase.
In Vivo Regulation of the LHCII Kinase by the Thiol Redox State in Chloroplasts.
Experiments presented in Fig. 4 were conducted with thylakoid membranes isolated from dark-adapted leaves and subsequently subjected to preillumination treatments. To study how the in vivo-activated or inactivated LHCII phosphorylation system reacts to changes in the thiol redox state, we next studied the thylakoid membranes isolated from low-light-treated leaves with active LHCII phosphorylation and from leaves illuminated at 1000 μmol photons·m−2·s−1 (HL) with inactive LHCII phosphorylation in our experimental conditions (Fig. 1) (9).
Under low-light illumination of leaves, the LHCII proteins are maximally phosphorylated. To study the regulation of LHCII phosphorylation in such thylakoids, we isolated the membranes in the absence of NaF, thus allowing partial dephosphorylation (ca. 60%) to occur during the isolation procedure. Rephosphorylation of LHCII proteins was subsequently studied in vitro (Table 1). LHCII phosphorylation was only slightly less sensitive to DTT-induced inhibition in these thylakoids compared with membranes isolated from dark-adapted leaves. Thus, the active state of LHCII kinase present in vivo at low light relaxes during the incubation of thylakoid membranes in darkness before the phosphorylation assay. Moreover, treatment of the thylakoids isolated from low-light-illuminated leaves with 0.1 mM NEM could not induce any significant loss in the capacity to phosphorylate LHCII proteins (Table 1), indicating that the regulatory thiols involved in the phosphorylation of LHCII proteins were in oxidized form in thylakoids isolated from low-light-illuminated leaves.
Table 1.
Inhibition of LHCII and D1 protein phosphorylation by DTT and NEM
| Inhibition of
phosphorylation, %*
|
||||
|---|---|---|---|---|
| Treatment of leaves | 2 mM DTT
|
0.1 mM NEM
|
||
| LHCII | D1 | LHCII | D1 | |
| Dark | 77 ± 5 | 14 ± 8 | 21 ± 5 | 7 ± 4 |
| LL | 69 ± 3 | 23 ± 9 | 28 ± 12 | 0 ± 11 |
| HL | 99 ± 3 | 11 ± 16 | 84 ± 7 | 2 ± 8 |
Thylakoid membranes were isolated from dark-adapted leaves (Dark) or from leaves illuminated at 30 (LL) or 1000 (HL) μmol photons·m−2·s−1 and subsequently incubated in the presence of DTT or NEM in darkness for 10 min, followed by phosphorylation for 30 min.
The percentage inhibition of LHCII and D1 protein phosphorylation (the means ± SD, n = 2–4) by a thiol reagent, as compared to a control assay without additions.
Thylakoid membranes isolated from high-light-illuminated leaves had a low capacity to phosphorylate LHCII proteins in vitro (see ref. 9). However, the capacity to phosphorylate LHCII proteins was totally restored during 10 min of incubation of the thylakoid membranes in darkness at room temperature (data not shown). Addition of DTT or NEM to the preincubation medium strongly prevented the restoration of LHCII phosphorylation capacity in the thylakoid membranes isolated from high-light-illuminated leaves (Table 1). Notably, in sharp contrast to thylakoids isolated from dark-adapted leaves (see Fig. 3), no pretreatment of these thylakoids with DTT was required to induce a strong inhibition of LHCII phosphorylation with NEM. These results indicate that the target groups involved in the regulation of LHCII phosphorylation by the thiol redox state are in a reduced sulfhydryl form in thylakoids isolated from high-light-illuminated leaves.
The low concentration of thiol reagents had no effect on the D1 phosphorylation capacity in thylakoids isolated from differentially illuminated leaves (Table 1).
Discussion
Phosphorylation of LHCII proteins in the thylakoid membrane is not only regulated by the cytochrome b6f complex-dependent activation of the LHCII kinase (5, 6, 20), but also by a specific mechanism mediated by the thiol redox state of chloroplasts (9, 10). Here we show that the chloroplastic thioredoxins f and m, which are reduced in light by ferredoxin-thioredoxin reductase (see refs. 21 and 22), are very effective inhibitors of LHCII phosphorylation in vitro (Fig. 2). Both thioredoxins induced a reduction of the target disulfide bond of the LHCII phosphorylation at micromolar concentrations, a result similar to that obtained with several thioredoxin-dependent chloroplastic enzymes (23–25).
Further insight into the mechanism of thiol inhibition of LHCII phosphorylation was obtained by applying NEM, a highly specific sulfhydryl group blocker (26), to the thylakoid membranes isolated from dark-adapted leaves. The regulatory thiol-disulfide groups involved in the inhibition of LHCII phosphorylation appeared to be oxidized in dark-adapted leaves and thus were not directly available to react with NEM. It should be noted that such a specific effect on LHCII phosphorylation was observed only when low concentrations of thiol modulators were used, indicating that the quantity of target molecules is low in the thylakoid membrane (see below). A general, nonspecific inhibition on PSII protein phosphorylation at high concentrations of NEM and DTT may result from steric hindrances in the interaction of the kinase with its effectors and/or substrates in thylakoid membranes (6, 7). This hindrance may also account for the total inhibition of thylakoid protein phosphorylation in the presence glutathione, which has a tendency to form mixed disulfides with protein thiols (see ref. 27).
How do the two apparently controversial regulation mechanisms of LHCII phosphorylation, activation via reduction of the cytochrome b6f complex and inactivation by reduction of a target disulfide bond, operate in vivo? One possibility is that these two regulatory mechanisms function independently. In this case, the cytochrome b6f complex-dependent activation of the kinase is in operation under all physiological light conditions, and the inactivation of LHCII phosphorylation via reduction of a target disulfide bond would be directly dependent on the concentration of reduced thiol redox mediators in the chloroplast. Our results, however, support a cooperative mode of regulation of LHCII phosphorylation with synergetic function of these two regulation mechanisms. Analogous dual regulation takes place in the chloroplast ATP synthase under dark–light transition (25, 28). In Fig. 5, a model is presented for the cooperative function of the two regulatory mechanisms of LHCII phosphorylation to reveal a distinct light-intensity-dependent steady-state pattern of LHCII phosphorylation observed under in vivo conditions.
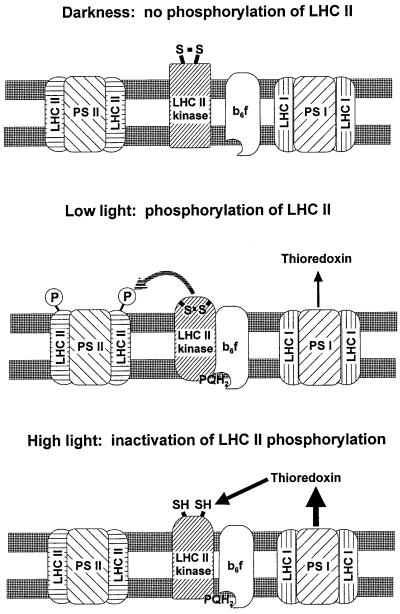
Figure 5.
Model for the regulation of LHCII phosphorylation in vivo. LHCII kinase is inactive in darkness because of the oxidized state of the Qo site in the cytochrome b6/f complex. Light initiates the electron transport in thylakoid membranes, resulting in activation of the LHCII kinase via binding of plastoquinol to the cytochrome b6/f complex. This activation induces a conformational change in the LHCII kinase with a concomitant burial and protection of the target regulatory disulfide bond against reduction. Such an active state prevails in chloroplasts under low light conditions. In high light, the regulatory disulfide bond becomes exposed and reduced via the ferredoxin–thioredoxin system, resulting in an inactive state of LHCII kinase despite the reduced state of the Qo site in cytochrome b6/f complex. The thicknesses of the black arrows indicate the amount of reduced thioredoxins under various light conditions.
Target Component in Thiol Redox Regulation of LHCII Phosphorylation.
Putative candidates to be modified by the thiol redox state include the LHCII kinase, the substrate proteins, and the cytochrome b6f complex. The low effective concentration (2 μM) of chloroplast thioredoxin f that was required to saturate the inhibition of LHCII phosphorylation suggests that target molecules are present in low concentrations in the thylakoid membrane. The estimated abundance of the LHCII kinase in the thylakoid membrane is lower by a factor of 100 or more than the concentrations of LHCII proteins and cytochrome b6f complex (6). The concentration of the substrate, LHCII proteins, can be roughly estimated by using a presumption that about half of the chlorophyll in the thylakoid membranes is associated with LHCII, and 12–14 chlorophylls are bound to each LHCII protein (6, 29). Using these values, we determined that the concentration of LHCII proteins is in the range 10–20 μM in our assay system. If LHCII proteins were specific targets for reduction by thioredoxin, the molar saturation ratio (mol of thioredoxin/mol of LHCII proteins) would be 0.1–0.2. Compared with much higher molar saturation ratios (above 4) reported for more specific systems employing purified chloroplast thioredoxins and target proteins (see ref. 24), it is very unlikely that the LHCII proteins are specific targets for thioredoxin. Thus we propose that the LHCII kinase, which occurs in thylakoid membranes at much lower concentrations, is regulated by a specific disulfide–sulfhydryl exchange in the enzyme molecule, in a way similar to that of the other thioredoxin-regulated chloroplastic enzymes (21, 22).
Model for Regulation of LHCII Phosphorylation at Physiological Light Intensities.
Three different regulatory states of the LHCII kinase are hypothesized to be involved in LHCII phosphorylation in vivo. Ambient light conditions determine the state of the LHCII kinase (Fig. 5). In dark-adapted leaves, the LHCII kinase is in a relaxed state. It is inactive because the regulatory site of the cytochrome b6f complex is not occupied by reduced plastoquinol (5, 20). Moreover, the regulatory thiols in the LHCII kinase are oxidized, as indicated by the experiments with low concentrations of NEM (Fig. 3). The disulfide bond, however, can be effectively reduced by DTT or thioredoxins (Fig. 2 and 3), indicating that it is exposed in the membrane. Reduction of the disulfide bond with DTT also made the LHCII kinase prone to inactivation by the subsequent addition of NEM (Fig. 3).
Light activates the LHCII kinase via reduction of plastoquinone and cytochrome b6f complex. This activation has been proposed to induce a conformational change in the LHCII kinase (11) and may simultaneously hide the regulatory disulfide bond, explaining the insensitivity of the enzyme to external thiol reagents in preilluminated thylakoid membranes (Fig. 4). Indeed, preincubation of thylakoid membranes in light at low temperature did not protect LHCII phosphorylation against inactivation with DTT (data not shown), supporting a conformational change in LHCII kinase occurring with the activation process.
We propose that reduction of plastoquinone either by light or by NADPH and ferredoxin in darkness (Fig. 4) induces a conformational change in the LHCII kinase with a concomitant burial of the target regulatory disulfide bond, resulting in activation of the enzyme (Fig. 5). This state of LHCII kinase prevails in chloroplasts in vivo at low irradiance, which induces maximal phosphorylation of LHCII proteins (Fig. 1).
Higher light intensities, which lead to inactivation of LHCII phosphorylation in vivo, induce the third regulatory state of the LHCII kinase. The regulatory disulfide bond becomes exposed again and is made accessible to reduction. This accessibility was clearly demonstrated by experiments with NEM: in thylakoids isolated from high-light-illuminated leaves, the regulatory thiols were in reduced form and could be directly blocked by NEM without any prereduction with DTT (Table 1). Why and how the regulatory site modulated by the thiol redox state becomes exposed in chloroplasts under high irradiances are not currently known. It is conceivable that a second conformational change in the enzyme induces this stable inactivation state of the LHCII kinase (Fig. 5). This interpretation is supported by the observation that the thylakoids isolated from high-light-illuminated leaves fully restore LHCII phosphorylation capacity during a short incubation at room temperature (data not shown) but not at low temperature (9) before the phosphorylation assay. In the former case, the recovery may be due to the relaxation of a conformational change with simultaneous oxidation of the regulatory thiols in the LHCII kinase by oxygen. This oxidation was completely inhibited when DTT was present in the preincubation medium (Table 1). Oxidation of both the chloroplastic thioredoxins and their target enzymes by oxygen in vitro has been reported (21).
In summary, we provide evidence that phosphorylation of LHCII proteins is regulated by a complicated network involving redox control both via plastoquinone and cytochrome b6f complex and via the thiol redox state of chloroplast. Moreover, these two regulatory systems are not independent but function cooperatively. Such a mechanism strictly couples the phosphorylation of LHCII proteins to the ambient redox state of chloroplasts, resulting in maximal phosphorylation of LHCII proteins at low light intensities. What could be the physiological significance of this strict regulation of LHCII phosphorylation in chloroplasts in vivo? Balancing of excitation energy between PSII and PSI is a classical role assigned to LHCII phosphorylation (reviewed, e.g., in ref. 4). It is not clear, however, what specific advantages this state transition, with phosphorylating conditions favoring PSI excitation and decreasing PSII excitation, would bring to the function of the photosynthetic apparatus at low-light conditions. Instead, specific acclimation processes that increase the light-harvesting capacity of PSII are initiated under decreasing growth irradiance (30, 31). Involvement of reversible LHCII phosphorylation in the photoacclimation of the photosynthetic apparatus, via sensing of the redox level of both plastoquinone and the thiol redox compounds in chloroplasts, is an important subject for study.
Acknowledgments
The authors thank Dr. Ana Chueca for a generous gift of chloroplast thioredoxins. This work was supported by the Academy of Finland, a European Union grant (ERB IC15-CT98-0126), and the Nordiskt Kontaktorgan för Jordbruksforskning.
Abbreviations
- D1
reaction center protein of photosystem II
- LHCII
chlorophyll a/b-binding proteins of photosystem II antenna
- NEM
N-ethylmaleimide
- P-
phosphorylated form of proteins
- PS
photosystem
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180054297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180054297
References
- 1.Bennett J. Nature (London) 1977;269:344–346. [Google Scholar]
- 2.Bennett J. FEBS Lett. 1979;103:342–344. doi: 10.1016/0014-5793(79)81358-7. [DOI] [PubMed] [Google Scholar]
- 3.Bennett J. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:281–311. [Google Scholar]
- 4.Allen J F. Biochim Biophys Acta. 1992;1098:275–335. doi: 10.1016/s0005-2728(09)91014-3. [DOI] [PubMed] [Google Scholar]
- 5.Vener A V, van Kan P J M, Rich P R, Ohad I, Andersson B. Proc Natl Acad Sci USA. 1997;94:1585–1590. doi: 10.1073/pnas.94.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gal A, Zer H, Ohad I. Physiol Plant. 1997;100:863–868. [Google Scholar]
- 7.Schuster G, Dewit M, Staehelin L A, Ohad I. J Cell Biol. 1986;103:71–80. doi: 10.1083/jcb.103.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett J, Shaw E K, Michel H. Eur J Biochem. 1988;171:95–100. doi: 10.1111/j.1432-1033.1988.tb13763.x. [DOI] [PubMed] [Google Scholar]
- 9.Rintamäki E, Salonen M, Suoranta U-M, Carlberg I, Andersson B, Aro E-M. J Biol Chem. 1997;272:30476–30482. doi: 10.1074/jbc.272.48.30476. [DOI] [PubMed] [Google Scholar]
- 10.Carlberg I, Rintamäki E, Aro E-M, Andersson B. Biochemistry. 1999;38:3197–3204. doi: 10.1021/bi982506o. [DOI] [PubMed] [Google Scholar]
- 11.Vener A V, Ohad I, Andersson B. Curr Opin Plant Biol. 1998;1:217–223. doi: 10.1016/s1369-5266(98)80107-6. [DOI] [PubMed] [Google Scholar]
- 12.Pursiheimo S, Rintamäki E, Baena-Gonzalez E, Aro E-M. FEBS Lett. 1998;423:178–182. doi: 10.1016/S0014-5793(98)00088-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rintamäki E, Kettunen R, Aro E-M. J Biol Chem. 1996;271:14870–14875. doi: 10.1074/jbc.271.25.14870. [DOI] [PubMed] [Google Scholar]
- 14.Larsson U K, Sundby C, Andersson B. Biochim Biophys Acta. 1987;894:59–68. [Google Scholar]
- 15.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Porra R J, Thompson W A, Kriedemann P E. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- 17.Islam K. Biochim Biophys Acta. 1987;893:333–341. [Google Scholar]
- 18.Baginsky S, Tiller K, Pfannschmidt T, Link G. Plant Mol Biol. 1999;39:1013–1023. doi: 10.1023/a:1006177807844. [DOI] [PubMed] [Google Scholar]
- 19.Zer H, Vink M, Keren N, Dilly-Hartwig H, Paulsen H, Herrmann R G, Andersson B, Ohad I. Proc Natl Acad Sci USA. 1999;96:8277–8282. doi: 10.1073/pnas.96.14.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zito F, Finazzi G, Delosme R, Nitschke W, Picot D, Wollman F-A. EMBO J. 1999;18:2961–2969. doi: 10.1093/emboj/18.11.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheibe R. Bot Acta. 1990;103:327–334. [Google Scholar]
- 22.Buchanan B B. Photosynth Res. 1992;33:147–162. doi: 10.1007/BF00039177. [DOI] [PubMed] [Google Scholar]
- 23.Braun H, Lichter A, Häberlein I. Eur J Biochem. 1996;240:781–788. doi: 10.1111/j.1432-1033.1996.0781h.x. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Jaramillo J, Chueca A, Jacquot J P, Hermoso R, Lazaro J J, Sahrawy M, Lopez-Gorge J. Plant Physiol. 1997;114:1169–1175. doi: 10.1104/pp.114.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz O, Schürmann P, Strotmann H. J Biol Chem. 1997;272:16924–16927. doi: 10.1074/jbc.272.27.16924. [DOI] [PubMed] [Google Scholar]
- 26.Zander T, Phadke N D, Bardwell J C A. Methods Enzymol. 1998;290:59–74. doi: 10.1016/s0076-6879(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert H F. Methods Enzymol. 1995;251:8–28. doi: 10.1016/0076-6879(95)51107-5. [DOI] [PubMed] [Google Scholar]
- 28.Shahak Y. J Biol Chem. 1985;260:1459–1464. [PubMed] [Google Scholar]
- 29.Kühlbrandt W, Wang D N, Fujiyoshi Y. Nature (London) 1994;367:614–621. doi: 10.1038/367614a0. [DOI] [PubMed] [Google Scholar]
- 30.Anderson J M, Aro E-M. Photosynth Res. 1994;41:315–326. doi: 10.1007/BF00019409. [DOI] [PubMed] [Google Scholar]
- 31.Escoubas J M, Lomas M, LaRoche J, Falkowsky G. Proc Natl Acad Sci USA. 1995;92:10234–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]