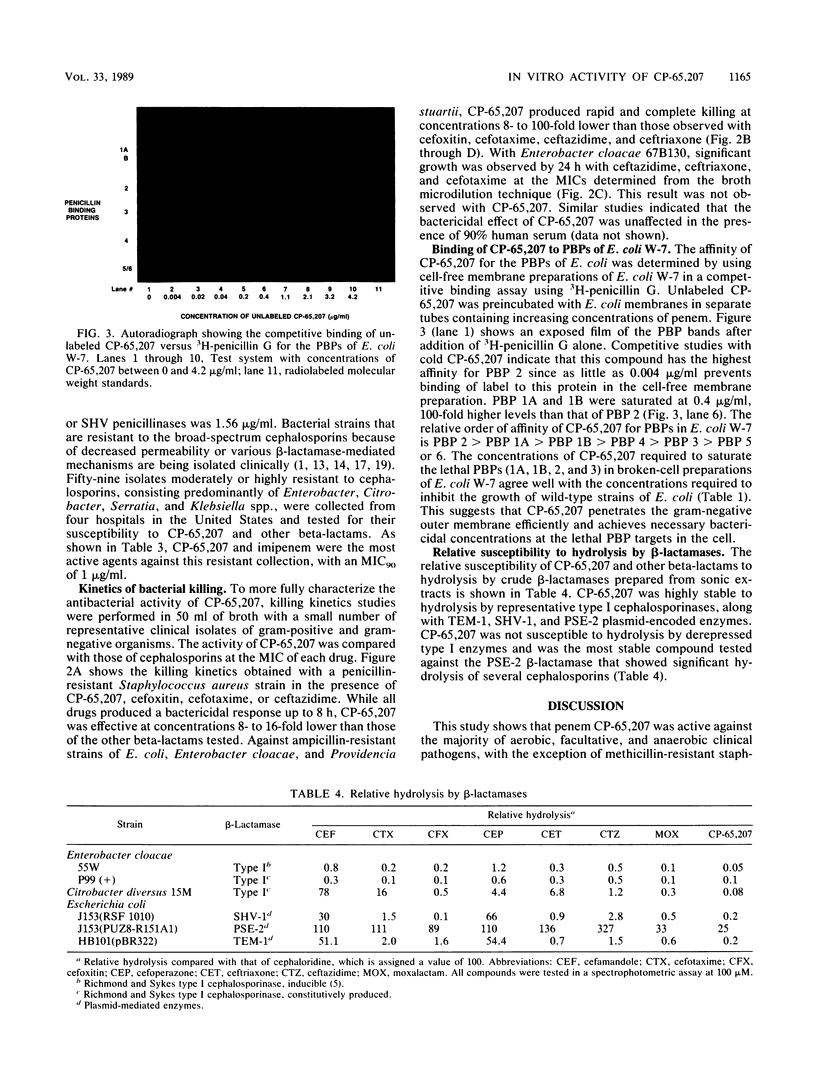
Abstract
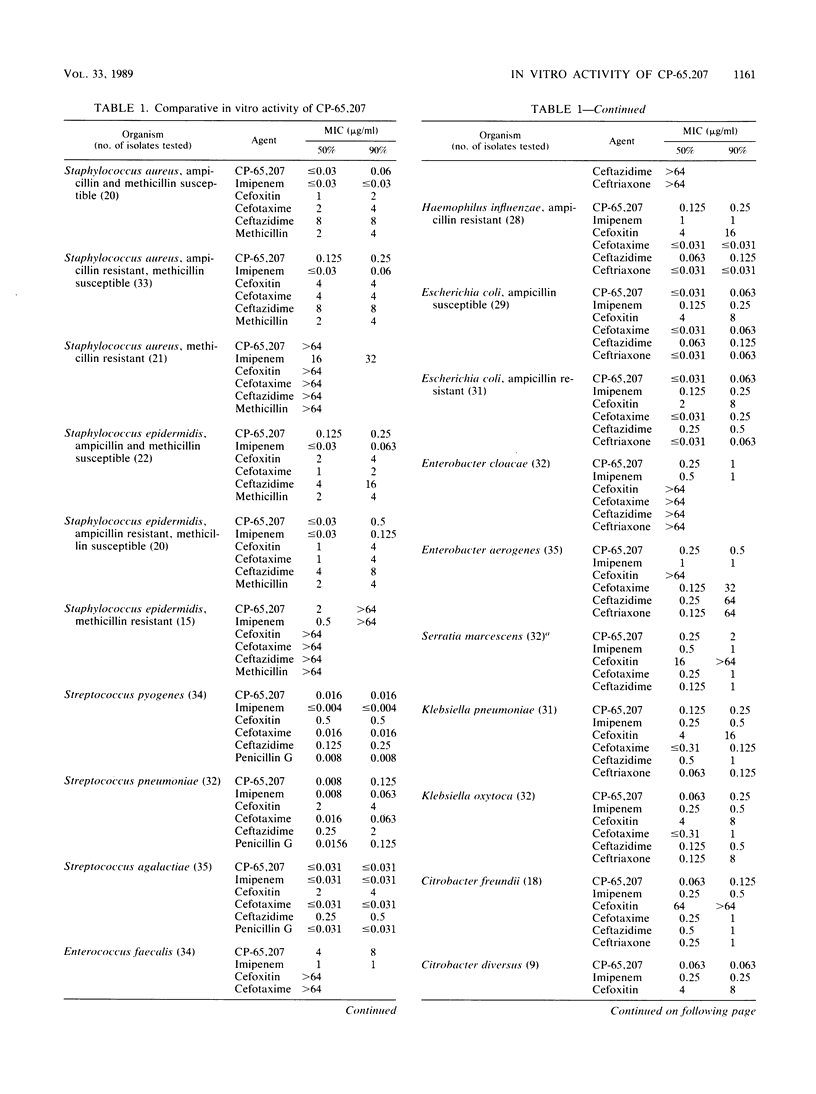
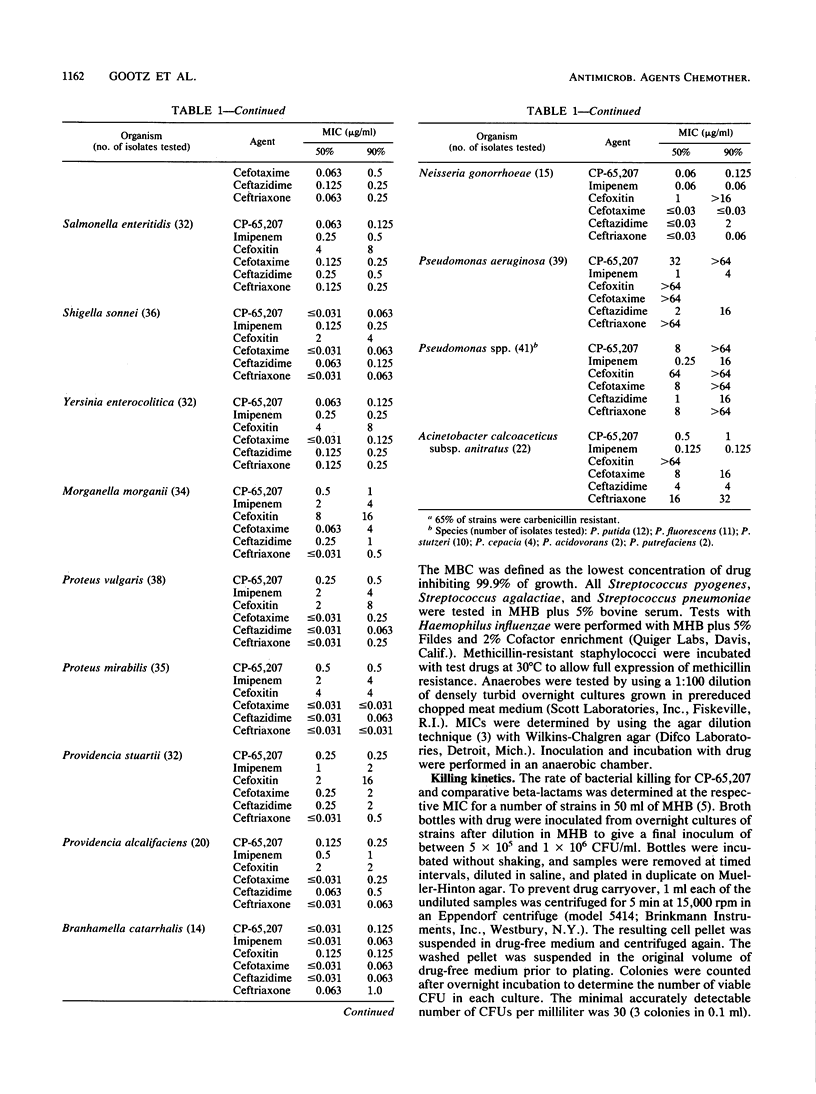
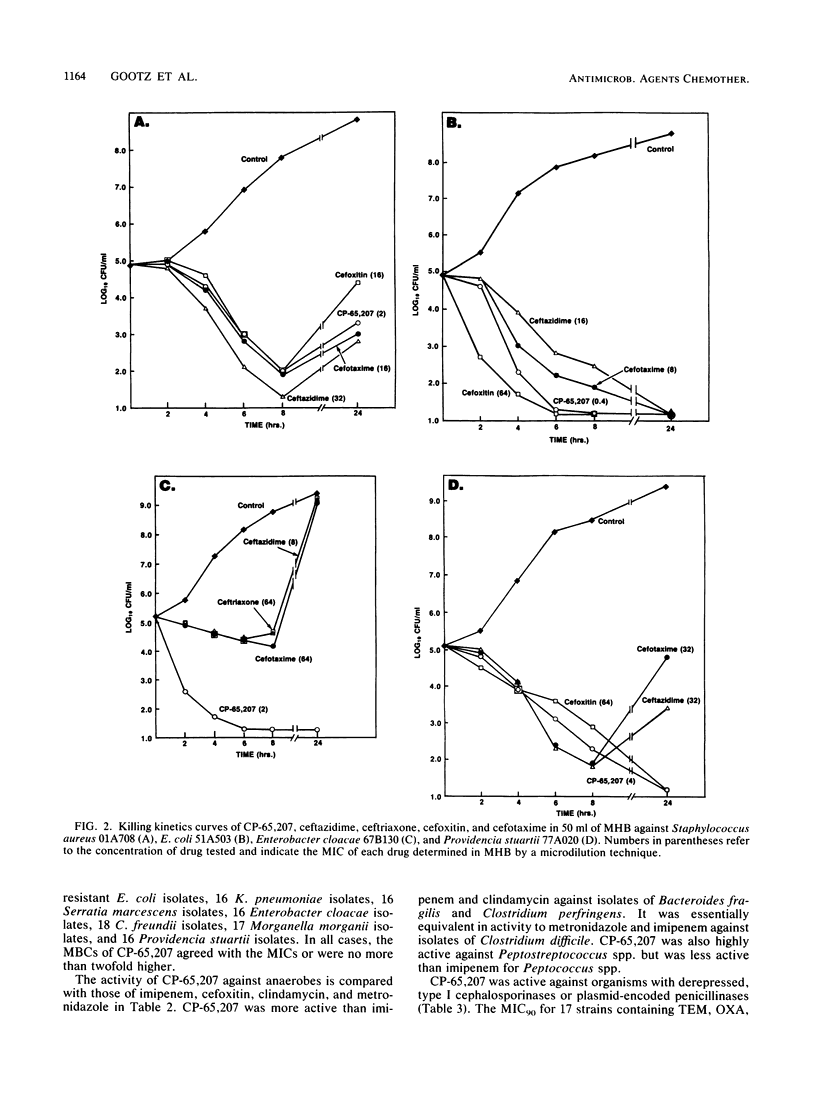
CP-65,207 is a new parenteral penem antibiotic with a broad spectrum that includes gram-positive, gram-negative, and anaerobic microorganisms, with MICs for 90% (MIC90s) of the majority of 1,101 clinical pathogens tested being less than or equal to 1 microgram/ml. The compound was from 10- to 100-fold more active than cefoxitin and broad-spectrum cephalosporins against gram-positive bacteria and anaerobes. CP-65,207 was less active than imipenem for staphylococci, group A streptococci, and Enterococcus faecalis. Against members of the family Enterobacteriaceae, CP-65,207 was in general 100-fold more active than cefoxitin, 5- to 10-fold more active than broad-spectrum cephalosporins, and 2-fold more active than imipenem. Fresh clinical isolates that were resistant to broad-spectrum cephalosporins were highly susceptible to CP-65,207 and imipenem (MIC90, 1 microgram/ml). Isolates of Enterococcus faecalis, Serratia marcescens, and anaerobic Peptococcus spp. had MIC90s of 8, 2, and 3.12 micrograms/ml, respectively. CP-65,207 was not very active against methicillin-resistant staphylococci or Pseudomonas aeruginosa. Killing kinetics showed that against some strains CP-65,207 is rapidly bactericidal at concentrations well below those required to achieve a similar degree of killing with cefotaxime, ceftazidime, and ceftriaxone. CP-65,207 was only slightly susceptible to hydrolysis by type I cephalosporinases and TEM-1, SHV-1, and PSE-2 plasmid-encoded enzymes. It had the highest affinity for penicillin-binding proteins 2, 1A, 1B, and 3 in cell-free preparations of Escherichia coli W-7.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckwith D. G., Jahre J. A. Role of a cefoxitin-inducible beta-lactamase in a case of breakthrough bacteremia. J Clin Microbiol. 1980 Oct;12(4):517–520. doi: 10.1128/jcm.12.4.517-520.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M., Wise R., Andrews J. M. Sch 29482--a novel penem antibiotic: an in-vitro comparison of its activity with other beta-lactams. J Antimicrob Chemother. 1982 Feb;9 (Suppl 100):17–23. doi: 10.1093/jac/9.suppl_c.17. [DOI] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C., Goering R. V. Resistance to cefamandole: derepression of beta-lactamases by cefoxitin and mutation in Enterobacter cloacae. J Infect Dis. 1982 Jul;146(1):34–42. doi: 10.1093/infdis/146.1.34. [DOI] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C., Sanders W. E., Jr In vitro activity of furazlocillin (Bay k 4999) compared with those of mezlocillin, piperacillin, and standard beta-lactam antibiotics. Antimicrob Agents Chemother. 1979 Jun;15(6):783–791. doi: 10.1128/aac.15.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan F. M., Kropp H., Sundelof J. G., Birnbaum J. Thienamycin: development of imipenen-cilastatin. J Antimicrob Chemother. 1983 Dec;12 (Suppl 500):1–35. doi: 10.1093/jac/12.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neu H. C., Chin N. X., Labthavikul P. The in-vitro activity of a novel penem FCE 22101 compared to other beta-lactam antibiotics. J Antimicrob Chemother. 1985 Sep;16(3):305–313. doi: 10.1093/jac/16.3.305. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chin N. X., Neu N. M. In vitro activity and beta-lactamase stability of a new penem, CGP 31608. Antimicrob Agents Chemother. 1987 Apr;31(4):558–569. doi: 10.1128/aac.31.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. Antibacterial activity of an oral penem, Sch 29482. J Antimicrob Chemother. 1982 Feb;9 (Suppl 100):49–57. doi: 10.1093/jac/9.suppl_c.49. [DOI] [PubMed] [Google Scholar]
- Norrby S. R., Jonsson M. Comparative in vitro antibacterial activity of Sch 34343, a novel penem antibiotic. Antimicrob Agents Chemother. 1985 Jan;27(1):128–131. doi: 10.1128/aac.27.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Moellering R. C., Jr, Martin R. R., Perkins R. L., Strike D. G., Gootz T. D., Sanders W. E., Jr Resistance to cefamandole: a collaborative study of emerging clinical problems. J Infect Dis. 1982 Jan;145(1):118–125. doi: 10.1093/infdis/145.1.118. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance during therapy with the newer beta-lactam antibiotics: role of inducible beta-lactamases and implications for the future. Rev Infect Dis. 1983 Jul-Aug;5(4):639–648. doi: 10.1093/clinids/5.4.639. [DOI] [PubMed] [Google Scholar]
- Seibert G., Isert D., Klesel N., Limbert M., Pries A., Schrinner E., Cooke M., Walmsley J., Bentley P. H. HRE 664, a new parenteral penem. I. Antibacterial activity in vitro. J Antibiot (Tokyo) 1987 May;40(5):660–667. doi: 10.7164/antibiotics.40.660. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Then R. L., Angehrn P. Trapping of nonhydrolyzable cephalosporins by cephalosporinases in Enterobacter cloacae and Pseudomonas aeruginosa as a possible resistance mechanism. Antimicrob Agents Chemother. 1982 May;21(5):711–717. doi: 10.1128/aac.21.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera P., Ernst F., Grenier P., Glupczynski Y., Husson M., Klastersky J. In-vitro activity of two new carbapenems FCE 22101 and CGP 31608 in comparison with imipenem. J Antimicrob Chemother. 1987 Aug;20(2):179–189. doi: 10.1093/jac/20.2.179. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Diffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985 Jan;27(1):84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak O., Lang M., Cozens R., Konopka E. A., Mett H., Schneider P., Tosch W., Scartazzini R. Penems: in vitro and in vivo experiments. J Clin Pharmacol. 1988 Feb;28(2):128–135. doi: 10.1002/j.1552-4604.1988.tb05736.x. [DOI] [PubMed] [Google Scholar]