Abstract
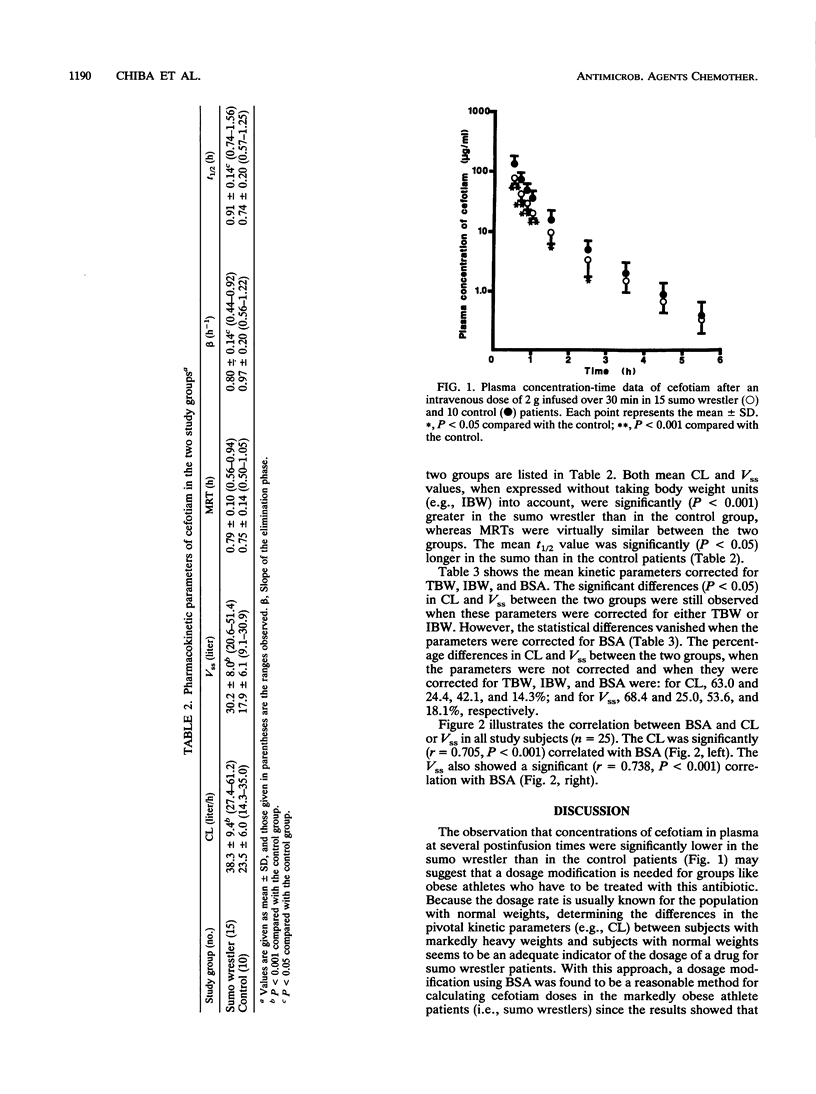
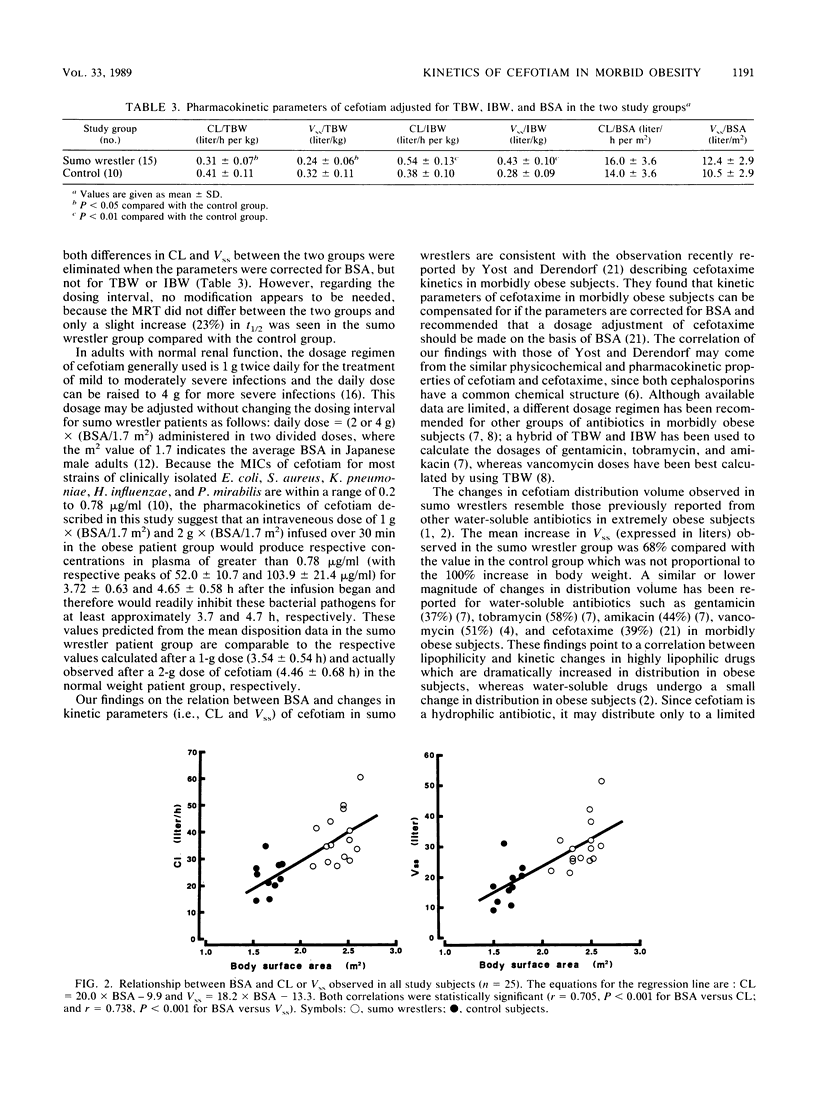
Markedly obese athletes like Japanese sumo wrestlers may frequently suffer various traumas which result in the prophylaxis or treatment of posttraumatic infection with antibiotics. However, appropriate dosage regimens in this group of patients have not been fully known for many antibiotics. Therefore, we studied the kinetic disposition of cefotiam, a parenteral, broad-spectrum cephalosporin with activity against gram-positive and -negative bacteria, after an intravenous dose (2 g) infused over 30 min into 15 sumo wrestler patients with an excess body weight (130 to 220% of ideal body weight) and 10 control patients with a normal weight (90 to 102% of ideal body weight). Mean (+/- standard deviation) clearance and steady-state volume of distribution were significantly greater in the sumo wrestler than in the control group (38.3 +/- 9.4 versus 23.5 +/- 6.0 liters/h, P less than 0.001, and 30.2 +/- 8.0 versus 17.9 +/- 6.1 liters, P less than 0.001). Mean elimination half-life was slightly but significantly longer in the sumo wrestler than in the control group (0.91 +/- 0.14 versus 0.74 +/- 0.20 h, P less than 0.05). However, mean residence time did not differ between the two groups (0.79 +/- 0.10 versus 0.75 +/- 0.14 h). The statistical differences in clearance and volume of distribution between the two groups disappeared when these kinetic parameters were corrected for body surface area, but not for total body weight or ideal body weight. The results suggest that the dosage calculation of cefotiam, a hydrophilic antibiotic, should be made on the basis of body surface area in morbidly obese athlete or sumo wrestler patients. However, whether this recommendation should extend to other nonathlete obese subjects remains to be determined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abernethy D. R., Greenblatt D. J., Divoll M., Harmatz J. S., Shader R. I. Alterations in drug distribution and clearance due to obesity. J Pharmacol Exp Ther. 1981 Jun;217(3):681–685. [PubMed] [Google Scholar]
- Abernethy D. R., Greenblatt D. J. Drug disposition in obese humans. An update. Clin Pharmacokinet. 1986 May-Jun;11(3):199–213. doi: 10.2165/00003088-198611030-00002. [DOI] [PubMed] [Google Scholar]
- Abernethy D. R., Greenblatt D. J. Pharmacokinetics of drugs in obesity. Clin Pharmacokinet. 1982 Mar-Apr;7(2):108–124. doi: 10.2165/00003088-198207020-00002. [DOI] [PubMed] [Google Scholar]
- Abernethy D. R., Greenblatt D. J., Smith T. W. Digoxin disposition in obesity: clinical pharmacokinetic investigation. Am Heart J. 1981 Oct;102(4):740–744. doi: 10.1016/0002-8703(81)90100-9. [DOI] [PubMed] [Google Scholar]
- Balant L., Dayer P., Auckenthaler R. Clinical pharmacokinetics of the third generation cephalosporins. Clin Pharmacokinet. 1985 Mar-Apr;10(2):101–143. doi: 10.2165/00003088-198510020-00001. [DOI] [PubMed] [Google Scholar]
- Bauer L. A., Edwards W. A., Dellinger E. P., Simonowitz D. A. Influence of weight on aminoglycoside pharmacokinetics in normal weight and morbidly obese patients. Eur J Clin Pharmacol. 1983;24(5):643–647. doi: 10.1007/BF00542215. [DOI] [PubMed] [Google Scholar]
- Blouin R. A., Bauer L. A., Miller D. D., Record K. E., Griffen W. O., Jr Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982 Apr;21(4):575–580. doi: 10.1128/aac.21.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin R. A., Mann H. J., Griffen W. O., Jr, Bauer L. A., Record K. E. Tobramycin pharmacokinetics in morbidly obese patients. Clin Pharmacol Ther. 1979 Oct;26(4):508–512. doi: 10.1002/cpt1979264508. [DOI] [PubMed] [Google Scholar]
- Bray G. A. Definition, measurement, and classification of the syndromes of obesity. Int J Obes. 1978;2(2):99–112. [PubMed] [Google Scholar]
- Brisson A. M., Bryskier A., Millerioux L., Fourtillan J. B. Pharmacokinetics of cefotiam administered intravenously and intramuscularly to healthy adults. Antimicrob Agents Chemother. 1984 Oct;26(4):513–518. doi: 10.1128/aac.26.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne R. E., Bauer L. A., Gibson G. A., Griffen W. O., Jr, Blouin R. A. Estimating creatinine clearance in morbidity obese patients. Am J Hosp Pharm. 1981 Jun;38(6):841–844. [PubMed] [Google Scholar]
- Rouan M. C., Binswanger U., Bammatter F., Theobald W., Schoeller J. P., Guibert J. Pharmacokinetics and dosage adjustment of cefotiam in renal impaired patients. J Antimicrob Chemother. 1984 Jun;13(6):611–618. doi: 10.1093/jac/13.6.611. [DOI] [PubMed] [Google Scholar]
- Rouan M. C., Lecaillon J. B., Guibert J., Modai J., Schoeller J. P. Pharmacokinetics of cefotiam in humans. Antimicrob Agents Chemother. 1985 Feb;27(2):177–180. doi: 10.1128/aac.27.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Kida M., Kondo M., Ono H., Takeuchi M., Nishi T. SCE-963, a new broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1978 Oct;14(4):557–568. doi: 10.1128/aac.14.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]
- Yost R. L., Derendorf H. Disposition of cefotaxime and its desacetyl metabolite in morbidly obese male and female subjects. Ther Drug Monit. 1986;8(2):189–194. doi: 10.1097/00007691-198606000-00011. [DOI] [PubMed] [Google Scholar]


