Abstract
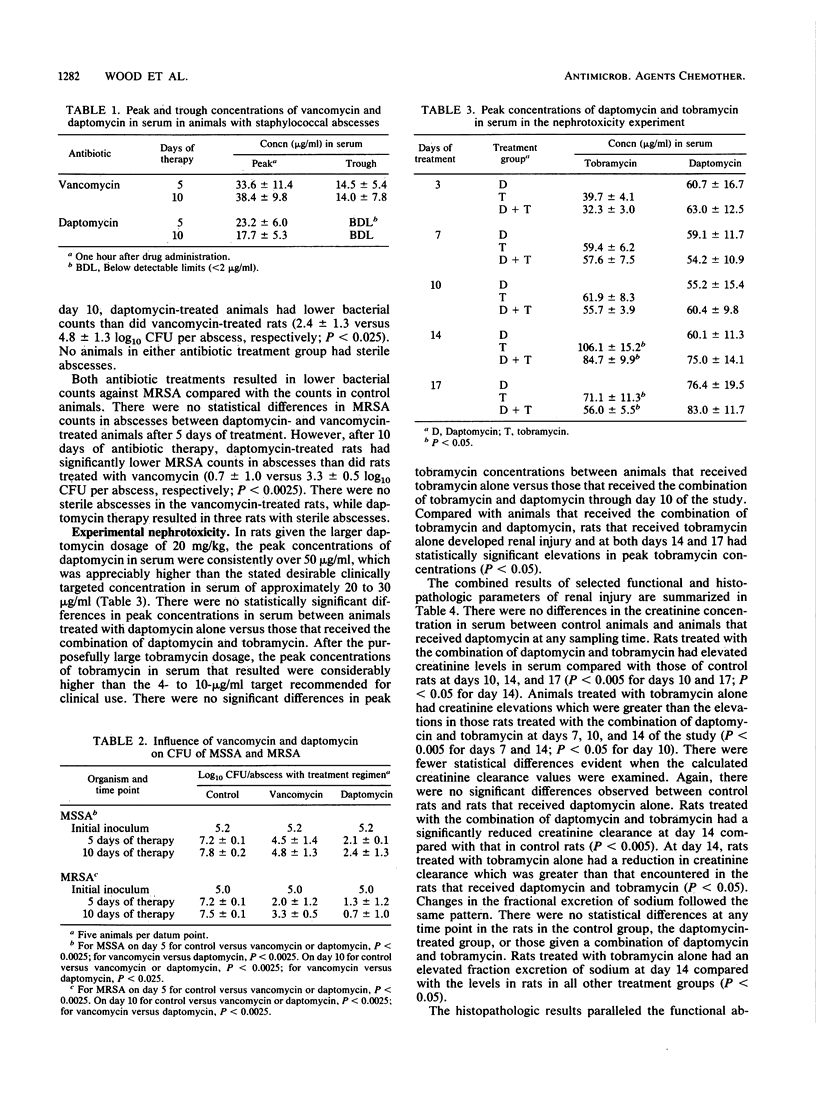
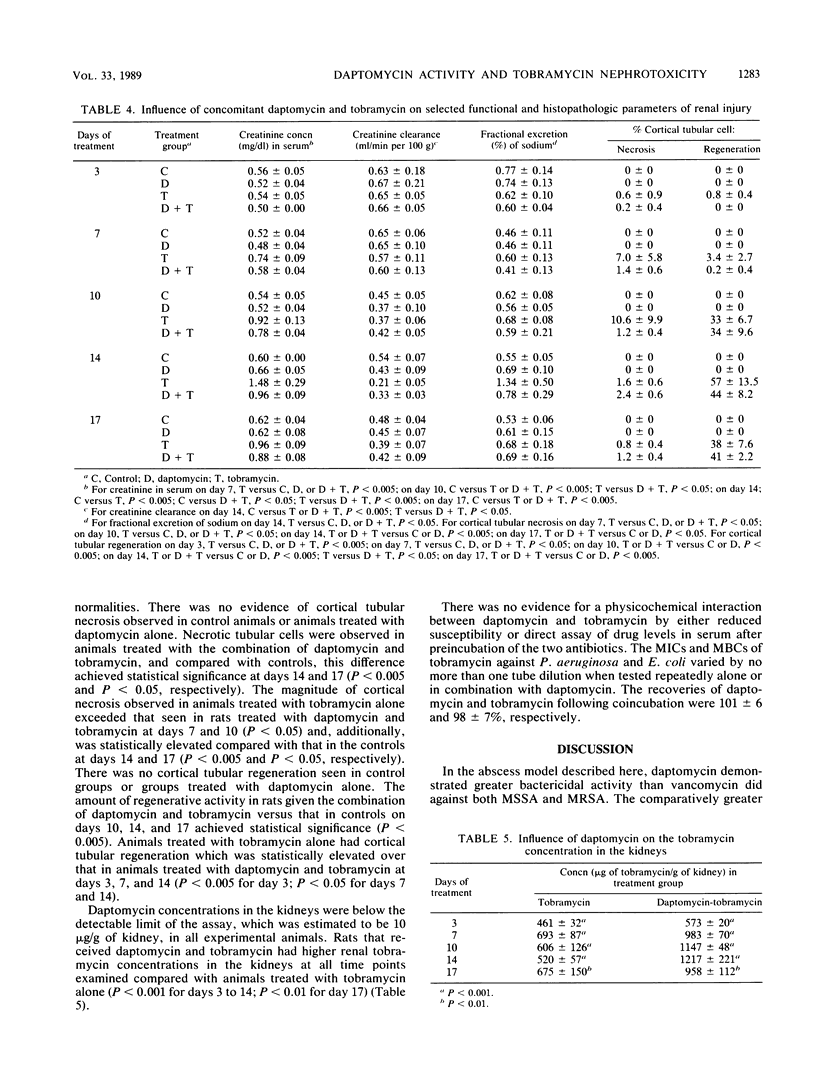
The antibacterial efficacies of daptomycin and vancomycin were compared in male Fischer rats with subcutaneous abscesses caused by either methicillin-susceptible Staphylococcus aureus (MSSA) or methicillin-resistant S. aureus (MRSA). The influence of daptomycin on tobramycin nephrotoxicity was also assessed. MSSA or MRSA abscesses were treated with subcutaneous daptomycin (10 mg/kg every 12 h), vancomycin (125 mg/kg every 12 h), or diluent (every 12 h) for 5 to 10 days. Rats in both antibiotic treatment groups had lower abscess bacterial counts than did controls at days 5 and 10 (P less than 0.0025). The daptomycin treatment groups had lower abscess bacterial counts than did the vancomycin treatment groups for MSSA at day 5 (P less than 0.0025) and day 10 (P less than 0.025) and for MRSA at day 10 (P less than 0.0025). Nephrotoxicity treatment groups included animals treated for 3, 7, 10, 14, and 17 days with subcutaneous diluent (every 12 h), daptomycin (20 mg/kg every 12 h), tobramycin (40 mg/kg every 12 h), and the combination of daptomycin and tobramycin. Compared with controls, animals treated with daptomycin alone exhibited no detectable nephrotoxicity. Rats given tobramycin alone developed functional and histopathologic abnormalities from days 7 through 17. Animals treated with daptomycin and tobramycin for 14 days had a lower mean concentration of creatinine in serum (P less than 0.005), higher mean creatinine clearance values (P less than 0.05), and less cortical tubular cell regeneration (P less than 0.05) than did rats treated with tobramycin alone. In rats with staphylococcal subcutaneous abscesses, daptomycin was superior to vancomycin in treating both MSSA and MRSA. Daptomycin alone caused no detectable renal injury, and in rats given daptomycin combined with tombramycin, there was less histologic and functional renal injury than in animals given tobramycin alone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. E., Hobbs J. N., Alborn W. E., Jr Inhibition of peptidoglycan biosynthesis in gram-positive bacteria by LY146032. Antimicrob Agents Chemother. 1987 Jul;31(7):1093–1099. doi: 10.1128/aac.31.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp D., Laurent G., Maldague P., Tulkens P. M. Reduction of gentamicin nephrotoxicity by the concomitant administration of poly-l-aspartic acid and poly-l-asparagine in rats. Arch Toxicol Suppl. 1986;9:306–309. doi: 10.1007/978-3-642-71248-7_52. [DOI] [PubMed] [Google Scholar]
- Benson C. A., Beaudette F., Trenholm G. Comparative in-vitro activity of LY146032 a new peptolide, with vancomycin and eight other agents against gram-positive organisms. J Antimicrob Chemother. 1987 Aug;20(2):191–196. doi: 10.1093/jac/20.2.191. [DOI] [PubMed] [Google Scholar]
- Bryant R. E., Mazza J. A., Gardner E. M. Effect of abscess milieu on bactericidal activity of LY146032 against staphylococci. Eur J Clin Microbiol. 1987 Apr;6(2):186–188. doi: 10.1007/BF02018206. [DOI] [PubMed] [Google Scholar]
- Coudron P. E., Johnston J. L., Archer G. L. In-vitro activity of LY146032 against Staphylococcus aureus and S. epidermidis. J Antimicrob Chemother. 1987 Oct;20(4):505–511. doi: 10.1093/jac/20.4.505. [DOI] [PubMed] [Google Scholar]
- De Broe M. E., Giuliano R. A., Verpooten G. A. Choice of drug and dosage regimen. Two important risk factors for aminoglycoside nephrotoxicity. Am J Med. 1986 Jun 30;80(6B):115–118. doi: 10.1016/0002-9343(86)90488-2. [DOI] [PubMed] [Google Scholar]
- Farber B. F., Moellering R. C., Jr Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother. 1983 Jan;23(1):138–141. doi: 10.1128/aac.23.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J., Helsel V. L. In vitro activity of LY146032 against staphylococci, streptococci, and enterococci. Antimicrob Agents Chemother. 1986 Nov;30(5):781–784. doi: 10.1128/aac.30.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. N., Wood C. A., Kohlhepp S. J., Kohnen P. W., Houghton D. C., Finkbeiner H. C., Lindsley J., Bennett W. M. Polyaspartic acid prevents experimental aminoglycoside nephrotoxicity. J Infect Dis. 1989 May;159(5):945–953. doi: 10.1093/infdis/159.5.945. [DOI] [PubMed] [Google Scholar]
- Huovinen P., Kotilainen P. In vitro activity of a new cyclic lipopeptide antibiotic, LY146032, against gram-positive clinical bacteria. Antimicrob Agents Chemother. 1987 Mar;31(3):455–457. doi: 10.1128/aac.31.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Onderdonk A. B., Gelfand J. A., Bartlett J. G., Gorbach S. L. A quantitative model for subcutaneous abscess formation in mice. Br J Exp Pathol. 1980 Feb;61(1):97–107. [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Antimicrobial activity and spectrum of LY146032, a lipopeptide antibiotic, including susceptibility testing recommendations. Antimicrob Agents Chemother. 1987 Apr;31(4):625–629. doi: 10.1128/aac.31.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kephart P. A., Esposito A. L. Comparison of the investigational drug, LY146032, with vancomycin in experimental pneumonia due to methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1988 Jan;21(1):33–39. doi: 10.1093/jac/21.1.33. [DOI] [PubMed] [Google Scholar]
- Pohlod D. J., Saravolatz L. D., Somerville M. M. In-vitro susceptibility of gram-positive cocci to LY146032 teicoplanin, sodium fusidate, vancomycin, and rifampicin. J Antimicrob Chemother. 1987 Aug;20(2):197–202. doi: 10.1093/jac/20.2.197. [DOI] [PubMed] [Google Scholar]
- Stratton C. W., Liu C., Weeks L. S. Activity of LY146032 compared with that of methicillin, cefazolin, cefamandole, cefuroxime, ciprofloxacin, and vancomycin against staphylococci as determined by kill-kinetic studies. Antimicrob Agents Chemother. 1987 Aug;31(8):1210–1215. doi: 10.1128/aac.31.8.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L. In vitro activity of LY146032, a new lipopeptide antibiotic, against gram-positive cocci. Antimicrob Agents Chemother. 1987 Feb;31(2):340–342. doi: 10.1128/aac.31.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat L. J., Smith J. W., Reynolds J., Bemis A. T., Treger T., Norton J. A. Comparison of cefazolin, cefamandole, vancomycin, and LY146032 for prophylaxis of experimental Staphylococcus epidermidis endocarditis. Antimicrob Agents Chemother. 1988 Jan;32(1):63–67. doi: 10.1128/aac.32.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. D., Hottendorf G. H., Bennett D. B. Inhibition of renal membrane binding and nephrotoxicity of aminoglycosides. J Pharmacol Exp Ther. 1986 Jun;237(3):919–925. [PubMed] [Google Scholar]
- Wood C. A., Kohlhepp S. J., Kohnen P. W., Houghton D. C., Gilbert D. N. Vancomycin enhancement of experimental tobramycin nephrotoxicity. Antimicrob Agents Chemother. 1986 Jul;30(1):20–24. doi: 10.1128/aac.30.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C. A., Norton D. R., Kohlhepp S. J., Kohnen P. W., Porter G. A., Houghton D. C., Brummett R. E., Bennett W. M., Gilbert D. N. The influence of tobramycin dosage regimens on nephrotoxicity, ototoxicity, and antibacterial efficacy in a rat model of subcutaneous abscess. J Infect Dis. 1988 Jul;158(1):13–22. doi: 10.1093/infdis/158.1.13. [DOI] [PubMed] [Google Scholar]


