Abstract
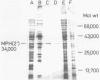
Macrolide 2'-phosphotransferase [MPH(2')] was purified 90-fold from an erythromycin-resistant strain of Escherichia coli, and its enzymatic properties were investigated. MPH(2') is an inducible intracellular enzyme which showed high levels of activity with 14-member-ring macrolides and extremely low levels with 16-member-ring macrolides. The optimum pH for inactivation of oleandomycin was 8.2, and the optimum temperature of the reaction was 40 degrees C. Enzyme activity was lost by heat treatment at 50 degrees C for 1 min. The isoelectric point and molecular weight of the enzyme were 5.3 and 34,000, respectively. Purine nucleotides, such as GTP, ITP, and ATP, were effective as cofactors in the inactivation of macrolides. Iodine, EDTA, or divalent cations inhibited MPH(2') activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andremont A., Gerbaud G., Courvalin P. Plasmid-mediated high-level resistance to erythromycin in Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):515–518. doi: 10.1128/aac.29.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andremont A., Raibaud P., Tancrède C. Effect of erythromycin on microbial antagonisms: a study in gnotobiotic mice associated with a human fecal flora. J Infect Dis. 1983 Sep;148(3):579–587. doi: 10.1093/infdis/148.3.579. [DOI] [PubMed] [Google Scholar]
- Andremont A., Tancrede C. Reduction of the aerobic Gram negative bacterial flora of the gastro-intestinal tract and prevention of traveller's diarrhea using oral erythromycin. Ann Microbiol (Paris) 1981 Nov-Dec;132 B(3):419–427. [PubMed] [Google Scholar]
- Arthur M., Andremont A., Courvalin P. Distribution of erythromycin esterase and rRNA methylase genes in members of the family Enterobacteriaceae highly resistant to erythromycin. Antimicrob Agents Chemother. 1987 Mar;31(3):404–409. doi: 10.1128/aac.31.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M., Courvalin P. Contribution of two different mechanisms to erythromycin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Nov;30(5):694–700. doi: 10.1128/aac.30.5.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélémy P., Autissier D., Gerbaud G., Courvalin P. Enzymic hydrolysis of erythromycin by a strain of Escherichia coli. A new mechanism of resistance. J Antibiot (Tokyo) 1984 Dec;37(12):1692–1696. doi: 10.7164/antibiotics.37.1692. [DOI] [PubMed] [Google Scholar]
- Brisson-Noël A., Arthur M., Courvalin P. Evidence for natural gene transfer from gram-positive cocci to Escherichia coli. J Bacteriol. 1988 Apr;170(4):1739–1745. doi: 10.1128/jb.170.4.1739-1745.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M., O'Hara K. Mechanisms of streptomycin(SM)-resistance of highly SM-resistant Pseudomonas aeruginosa strains. J Antibiot (Tokyo) 1976 Feb;29(2):169–175. doi: 10.7164/antibiotics.29.169. [DOI] [PubMed] [Google Scholar]
- Kono M., O'Hara K., Nagawa M., Mitsuhashi S. Drug resistance of staphylococci. Ability of chloramphenicol related compounds to induce chloramphenicol resistance in Staphylococcus aureus. Jpn J Microbiol. 1971 May;15(3):219–227. doi: 10.1111/j.1348-0421.1971.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Kono M., O'Hara K., Ohmiya K., Iida H., Kibayashi C., Kasahara K. [Antibacterial activity of (+)-negamycin prepared by a new method]. Jpn J Antibiot. 1986 Jan;39(1):247–249. [PubMed] [Google Scholar]
- Kono M., O'Hara K., Shiomi Y. Nuclear magnetic resonance spectrometric assay of beta-lactamase. Antimicrob Agents Chemother. 1980 Jan;17(1):16–19. doi: 10.1128/aac.17.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marshall V. P., Cialdella J. I., Baczynskyj L., Liggett W. F., Johnson R. A. Microbial O-phosphorylation of macrolide antibiotics. J Antibiot (Tokyo) 1989 Jan;42(1):132–134. doi: 10.7164/antibiotics.42.132. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Chou J. Release of surface enzymes in Enterobacteriaceae by osmotic shock. J Bacteriol. 1967 Dec;94(6):1934–1945. doi: 10.1128/jb.94.6.1934-1945.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura T., Kawada Y., Shiomi Y., O'Hara K., Kono M. Microbial degradation of cephalothin by cephalothin-susceptible Escherichia coli. Antimicrob Agents Chemother. 1978 Jun;13(6):1036–1039. doi: 10.1128/aac.13.6.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara K., Kanda T., Kono M. Structure of a phosphorylated derivative of oleandomycin, obtained by reaction of oleandomycin with an extract of an erythromycin-resistant strain of Escherichia coli. J Antibiot (Tokyo) 1988 Jun;41(6):823–827. doi: 10.7164/antibiotics.41.823. [DOI] [PubMed] [Google Scholar]
- O'Hara K., Kono M., Mitsuhashi S. Enzymatic inactivation of a new aminoglycoside antibiotic, sisomicin by resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Jun;5(6):558–561. doi: 10.1128/aac.5.6.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounissi H., Courvalin P. Nucleotide sequence of the gene ereA encoding the erythromycin esterase in Escherichia coli. Gene. 1985;35(3):271–278. doi: 10.1016/0378-1119(85)90005-8. [DOI] [PubMed] [Google Scholar]
- Wiley P. F., Baczynskyj L., Dolak L. A., Cialdella J. I., Marshall V. P. Enzymatic phosphorylation of macrolide antibiotics. J Antibiot (Tokyo) 1987 Feb;40(2):195–201. doi: 10.7164/antibiotics.40.195. [DOI] [PubMed] [Google Scholar]
- Yamagishi S., O'Hara K., Sawai T., Mitsuhashi S. The purification and properties of penicillin beta-lactamases mediated by transmissible R factors in Escherichia coli. J Biochem. 1969 Jul;66(1):11–20. doi: 10.1093/oxfordjournals.jbchem.a129111. [DOI] [PubMed] [Google Scholar]