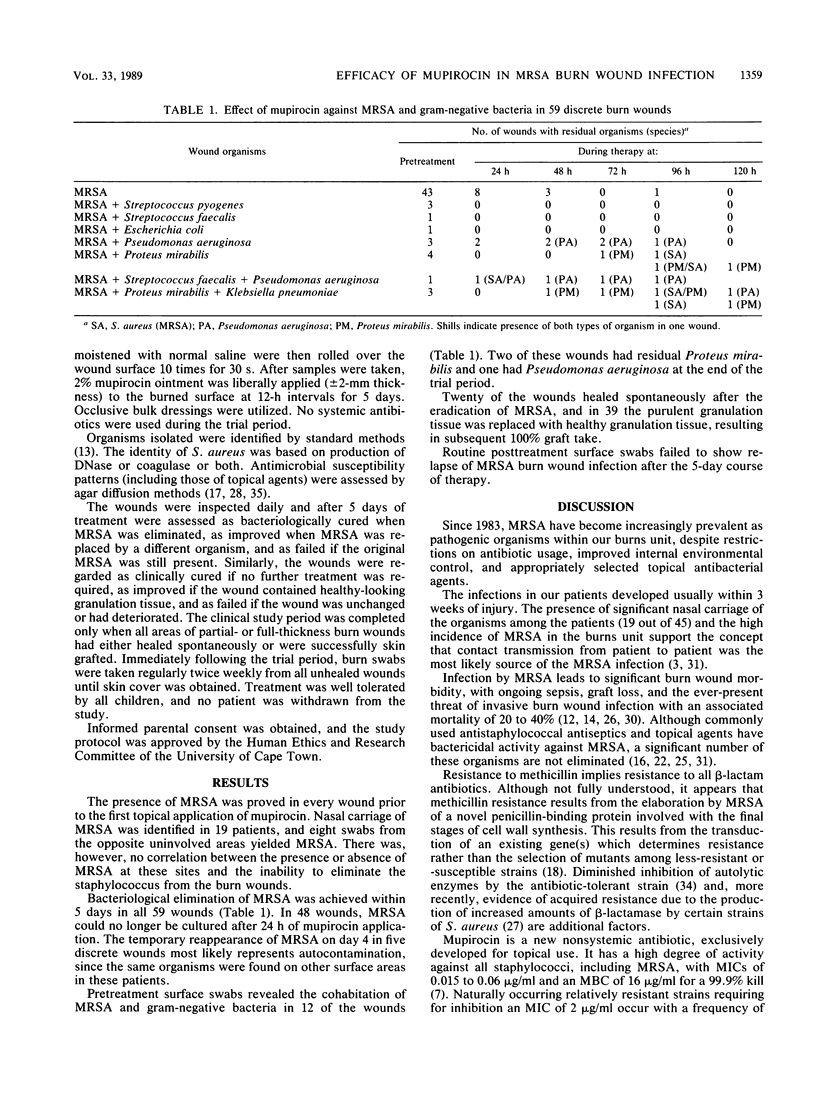
Abstract
Methicillin-resistant Staphylococcus aureus strains (MRSA) have become increasingly prevalent as nosocomial pathogens, especially in burn wounds. MRSA constituted 38% of all S. aureus isolates in our 25-bed burns unit despite the utilization of a combination of 1% silver sulfadiazine and 0.2% chlorhexidine as topical therapy. Mupirocin, a new antibiotic, has proved in vitro and in vivo to be highly effective in the treatment of MRSA infections. A prospective clinical trial with mupirocin ointment in MRSA burn wound infection was untertaken. Forty-five children with 59 discrete burn wounds and from whom MRSA were isolated were treated with 2% mupirocin ointment under occlusive dressings, applied twice daily for 5 days. The average burned area treated was 8% (range, 2 to 20%) of the total body surface area. The burn wounds were assessed clinically and bacteriologically daily. Mupirocin eliminated MRSA in all 59 wounds treated, with the maximum therapeutic response seen within 4 days. In three wounds, gram-negative organisms persisted after 5 days of topical therapy. Treatment was well tolerated by all children. We recommend that mupirocin in its present polyethylene glycol base should be used only on a selective basis, when current prophylactic topical therapy has failed to control MRSA infection in burns of less than 20% of the total body surface area, and that it should be applied only for a limited period of 5 days. The safety and the efficacy of mupirocin in burns exceeding 20% of the total body surface area need to be established.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayliffe G. A., Green W., Livingston R., Lowbury E. J. Antibiotic-resistant Staphylococcus aureus in dermatology and burn wards. J Clin Pathol. 1977 Jan;30(1):40–44. doi: 10.1136/jcp.30.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokas C. A., Paton J. H., Gibson M. F., Graham F., McLoughlin G. A., Croton R. S. Control and eradication of methicillin-resistant Staphylococcus aureus on a surgical unit. N Engl J Med. 1984 Nov 29;311(22):1422–1425. doi: 10.1056/NEJM198411293112207. [DOI] [PubMed] [Google Scholar]
- Boyce J. M., Landry M., Deetz T. R., DuPont H. L. Epidemiologic studies of an outbreak of nosocomial methicillin-resistant Staphylococcus aureus infections. Infect Control. 1981 Mar-Apr;2(2):110–116. doi: 10.1017/s0195941700053881. [DOI] [PubMed] [Google Scholar]
- Bradley J. M., Noone P., Townsend D. E., Grubb W. B. Methicillin-resistant Staphylococcus aureus in a London hospital. Lancet. 1985 Jun 29;1(8444):1493–1495. doi: 10.1016/s0140-6736(85)92263-9. [DOI] [PubMed] [Google Scholar]
- Bruns D. E., Herold D. A., Rodeheaver G. T., Edlich R. F. Polyethylene glycol intoxication in burn patients. Burns Incl Therm Inj. 1982 Sep;9(1):49–52. doi: 10.1016/0305-4179(82)90136-x. [DOI] [PubMed] [Google Scholar]
- Casewell M. W., Hill R. L. In-vitro activity of mupirocin ('pseudomonic acid') against clinical isolates of Staphylococcus aureus. J Antimicrob Chemother. 1985 May;15(5):523–531. doi: 10.1093/jac/15.5.523. [DOI] [PubMed] [Google Scholar]
- Casewell M. W., Hill R. L. Mupirocin ('pseudomonic acid')--a promising new topical antimicrobial agent. J Antimicrob Chemother. 1987 Jan;19(1):1–5. doi: 10.1093/jac/19.1.1. [DOI] [PubMed] [Google Scholar]
- Chirife J., Herszage L., Joseph A., Bozzini J. P., Leardini N., Kohn E. S. In vitro antibacterial activity of concentrated polyethylene glycol 400 solutions. Antimicrob Agents Chemother. 1983 Sep;24(3):409–412. doi: 10.1128/aac.24.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. M. Topical use of silver sulphadiazine and chlorhexidine in the prevention of infection in thermal injuries. Med J Aust. 1975 Mar 29;1(13):413–415. [PubMed] [Google Scholar]
- Collopy B. T., Dalton M. F., Wright C., Mullany C. Comparison of the clinical significance of methicillin-resistant and methicillin-sensitive Staphylococcus aureus isolations. Med J Aust. 1984 Feb 18;140(4):211–214. doi: 10.5694/j.1326-5377.1984.tb103997.x. [DOI] [PubMed] [Google Scholar]
- Crossley K., Loesch D., Landesman B., Mead K., Chern M., Strate R. An outbreak of infections caused by strains of Staphylococcus aureus resistant to methicillin and aminoglycosides. I. Clinical studies. J Infect Dis. 1979 Mar;139(3):273–279. doi: 10.1093/infdis/139.3.273. [DOI] [PubMed] [Google Scholar]
- Fuller A. T., Mellows G., Woolford M., Banks G. T., Barrow K. D., Chain E. B. Pseudomonic acid: an antibiotic produced by Pseudomonas fluorescens. Nature. 1971 Dec 17;234(5329):416–417. doi: 10.1038/234416a0. [DOI] [PubMed] [Google Scholar]
- Haley C. E., Marling-Cason M., Smith J. W., Luby J. P., Mackowiak P. A. Bactericidal activity of antiseptics against methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985 Jun;21(6):991–992. doi: 10.1128/jcm.21.6.991-992.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneine N., Stewart P. R. Physiological determination of methicillin resistance in Staphylococcus aureus: comparison of clinical and genetically derived isolates. J Antimicrob Chemother. 1986 Jun;17(6):705–715. doi: 10.1093/jac/17.6.705. [DOI] [PubMed] [Google Scholar]
- Hughes J., Mellows G. On the mode of action of pseudomonic acid: inhibition of protein synthesis in Staphylococcus aureus. J Antibiot (Tokyo) 1978 Apr;31(4):330–335. doi: 10.7164/antibiotics.31.330. [DOI] [PubMed] [Google Scholar]
- Inman R. J., Snelling C. F., Roberts F. J., Shaw K., Boyle J. C. Prospective comparison of silver sulfadiazine 1 per cent plus chlorhexidine digluconate 0.2 per cent (Silvazine) and silver sulfadiazine 1 per cent (Flamazine) as prophylaxis against burn wound infection. Burns Incl Therm Inj. 1984 Oct;11(1):35–40. doi: 10.1016/0305-4179(84)90159-1. [DOI] [PubMed] [Google Scholar]
- Jarvis W. R., Thornsberry C., Boyce J., Hughes J. M. Methicillin-resistant Staphylococcus aureus at children's hospitals in the United States. Pediatr Infect Dis. 1985 Nov-Dec;4(6):651–655. doi: 10.1097/00006454-198511000-00011. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Keyworth N., Lincoln C. Staphylococci in the U.K.: a review. J Antimicrob Chemother. 1984 Dec;14 (Suppl 500):19–25. doi: 10.1093/jac/14.suppl_d.19. [DOI] [PubMed] [Google Scholar]
- Lawrence J. C., Cason J. S., Kidson A. Evaluation of phenoxetol-chlorhexidine cream as a prophylactic antibacterial agent in burns. Lancet. 1982 May 8;1(8280):1037–1040. doi: 10.1016/s0140-6736(82)92098-0. [DOI] [PubMed] [Google Scholar]
- Linnemann C. C., Jr, Mason M., Moore P., Korfhagen T. R., Staneck J. L. Methicillin-resistant Staphylococcus aureus: experience in a general hospital over four years. Am J Epidemiol. 1982 Jun;115(6):941–950. doi: 10.1093/oxfordjournals.aje.a113381. [DOI] [PubMed] [Google Scholar]
- McDougal L. K., Thornsberry C. The role of beta-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J Clin Microbiol. 1986 May;23(5):832–839. doi: 10.1128/jcm.23.5.832-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mupirocin-resistant Staphylococcus aureus. Lancet. 1987 Aug 15;2(8555):387–388. [PubMed] [Google Scholar]
- Parry M. F., Wallach R. Ethylene glycol poisoning. Am J Med. 1974 Jul;57(1):143–150. doi: 10.1016/0002-9343(74)90780-3. [DOI] [PubMed] [Google Scholar]
- Pavillard R., Harvey K., Douglas D., Hewstone A., Andrew J., Collopy B., Asche V., Carson P., Davidson A., Gilbert G. Epidemic of hospital-acquired infection due to methicillin-resistant Staphylococcus aureus in major Victorian hospitals. Med J Aust. 1982 May 29;1(11):451–454. [PubMed] [Google Scholar]
- Peacock J. E., Jr, Marsik F. J., Wenzel R. P. Methicillin-resistant Staphylococcus aureus: introduction and spread within a hospital. Ann Intern Med. 1980 Oct;93(4):526–532. doi: 10.7326/0003-4819-93-4-526. [DOI] [PubMed] [Google Scholar]
- Rode H., de Wet P. M., Millar A. J., Cywes S. Bactericidal efficacy of mupirocin in multi-antibiotic resistant Staphylococcus aureus burn wound infection. J Antimicrob Chemother. 1988 May;21(5):589–595. doi: 10.1093/jac/21.5.589. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Wheeler N., Laverdiere M., Blazevic D., Wilkinson B. J. A new type of penicillin resistance of Staphylococcus aureus. Lancet. 1977 Feb 26;1(8009):443–447. doi: 10.1016/s0140-6736(77)91941-9. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Boon R. J., Griffin K. E., Masters P. J., Slocombe B., White A. R. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother. 1985 Apr;27(4):495–498. doi: 10.1128/aac.27.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. F. Choice of chemotherapy for infection by Staphylococcus aureus. J Antimicrob Chemother. 1982 Jan;9(1):1–3. doi: 10.1093/jac/9.1.1. [DOI] [PubMed] [Google Scholar]
- Wuite J., Davies B. I., Go M., Lambers J., Jackson D., Mellows G. Pseudomonic acid: a new topical antimicrobial agent. Lancet. 1983 Aug 13;2(8346):394–394. doi: 10.1016/s0140-6736(83)90358-6. [DOI] [PubMed] [Google Scholar]


