Abstract
The interaction between ciprofloxacin and enoxacin and phospholipid-containing bilayers was examined as the initial step in transmembrane diffusion processes. By using cosedimentation, maximal association of liposomes and 14C-labeled enoxacin and ciprofloxacin was detected at acidic and neutral pHs. Aqueous solubility of ciprofloxacin, enoxacin, and norfloxacin was poorest at neutral pH and greater at alkaline or acidic pHs. These investigations suggest that the interaction occurs because of ionic and hydrophobic forces and is nonsaturable up to 20 micrograms/ml.
Full text
PDF
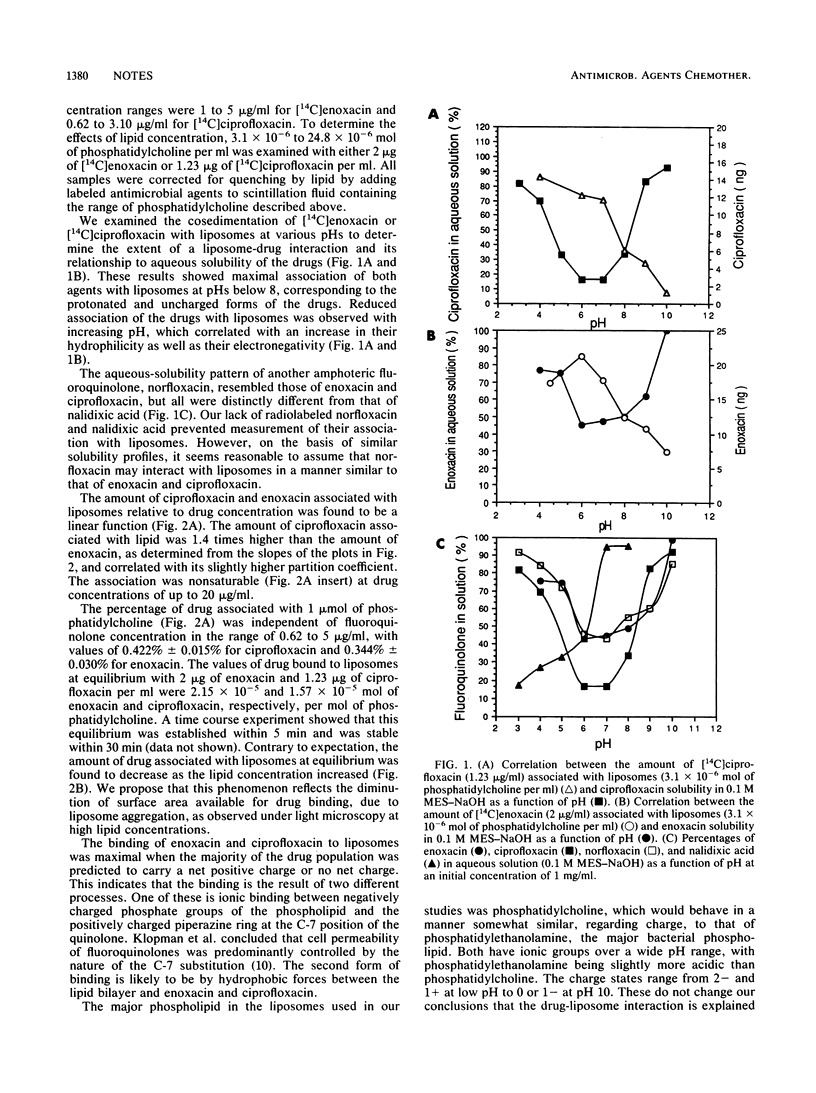
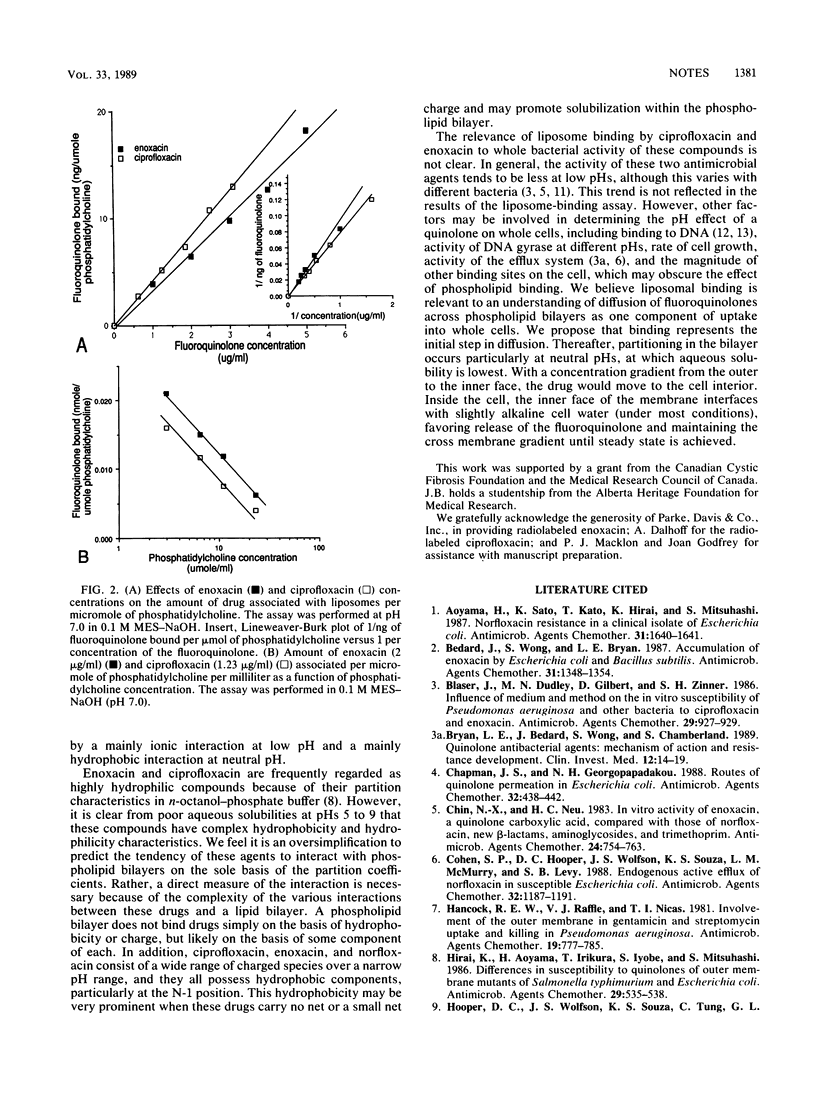

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama H., Sato K., Kato T., Hirai K., Mitsuhashi S. Norfloxacin resistance in a clinical isolate of Escherichia coli. Antimicrob Agents Chemother. 1987 Oct;31(10):1640–1641. doi: 10.1128/aac.31.10.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard J., Wong S., Bryan L. E. Accumulation of enoxacin by Escherichia coli and Bacillus subtilis. Antimicrob Agents Chemother. 1987 Sep;31(9):1348–1354. doi: 10.1128/aac.31.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser J., Dudley M. N., Gilbert D., Zinner S. H. Influence of medium and method on the in vitro susceptibility of Pseudomonas aeruginosa and other bacteria to ciprofloxacin and enoxacin. Antimicrob Agents Chemother. 1986 May;29(5):927–929. doi: 10.1128/aac.29.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Bedard J., Wong S., Chamberland S. Quinolone antimicrobial agents: mechanism of action and resistance development. Clin Invest Med. 1989 Feb;12(1):14–19. [PubMed] [Google Scholar]
- Chapman J. S., Georgopapadakou N. H. Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother. 1988 Apr;32(4):438–442. doi: 10.1128/aac.32.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. In vitro activity of enoxacin, a quinolone carboxylic acid, compared with those of norfloxacin, new beta-lactams, aminoglycosides, and trimethoprim. Antimicrob Agents Chemother. 1983 Nov;24(5):754–763. doi: 10.1128/aac.24.5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., Hooper D. C., Wolfson J. S., Souza K. S., McMurry L. M., Levy S. B. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1187–1191. doi: 10.1128/aac.32.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Raffle V. J., Nicas T. I. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981 May;19(5):777–785. doi: 10.1128/aac.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopman G., Macina O. T., Levinson M. E., Rosenkranz H. S. Computer automated structure evaluation of quinolone antibacterial agents. Antimicrob Agents Chemother. 1987 Nov;31(11):1831–1840. doi: 10.1128/aac.31.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves D. S., Bywater M. J., Holt H. A., White L. O. In-vitro studies with ciprofloxacin, a new 4-quinolone compound. J Antimicrob Chemother. 1984 Apr;13(4):333–346. doi: 10.1093/jac/13.4.333. [DOI] [PubMed] [Google Scholar]
- Shen L. L., Pernet A. G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: the target of the drugs is DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):307–311. doi: 10.1073/pnas.82.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornaletti S., Pedrini A. M. Studies on the interaction of 4-quinolones with DNA by DNA unwinding experiments. Biochim Biophys Acta. 1988 Mar 31;949(3):279–287. doi: 10.1016/0167-4781(88)90153-4. [DOI] [PubMed] [Google Scholar]


