Abstract
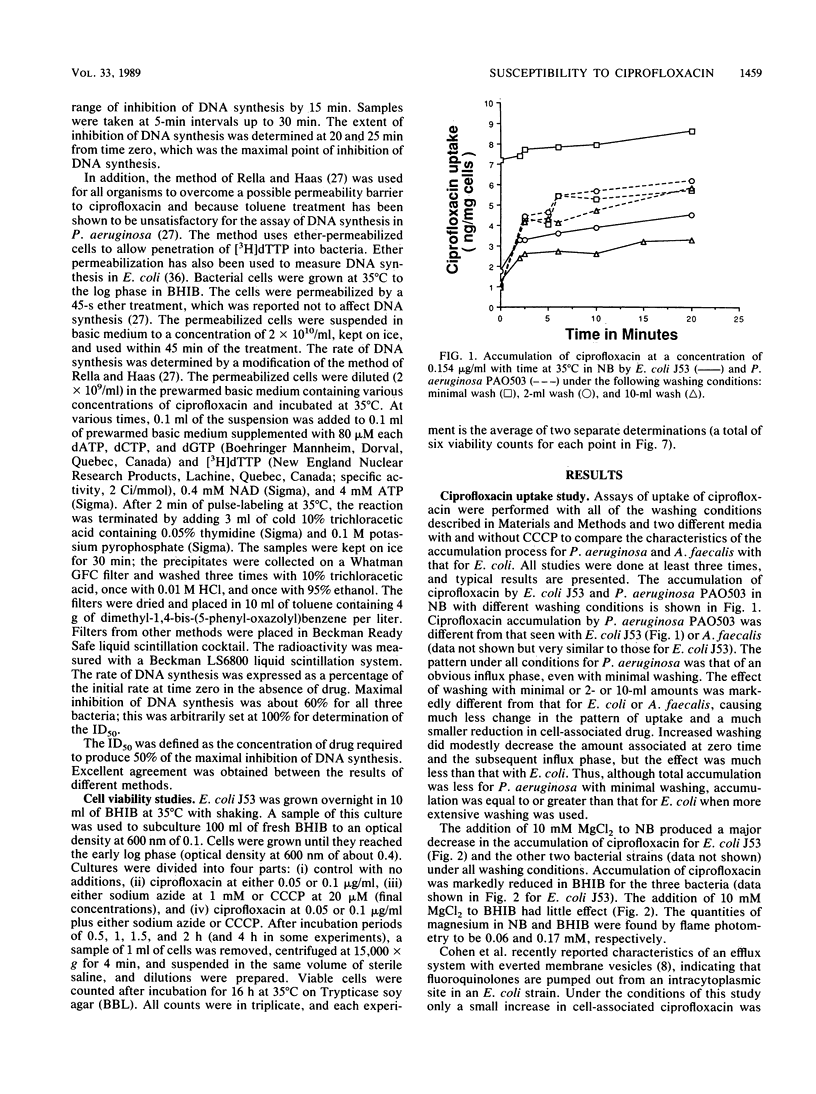
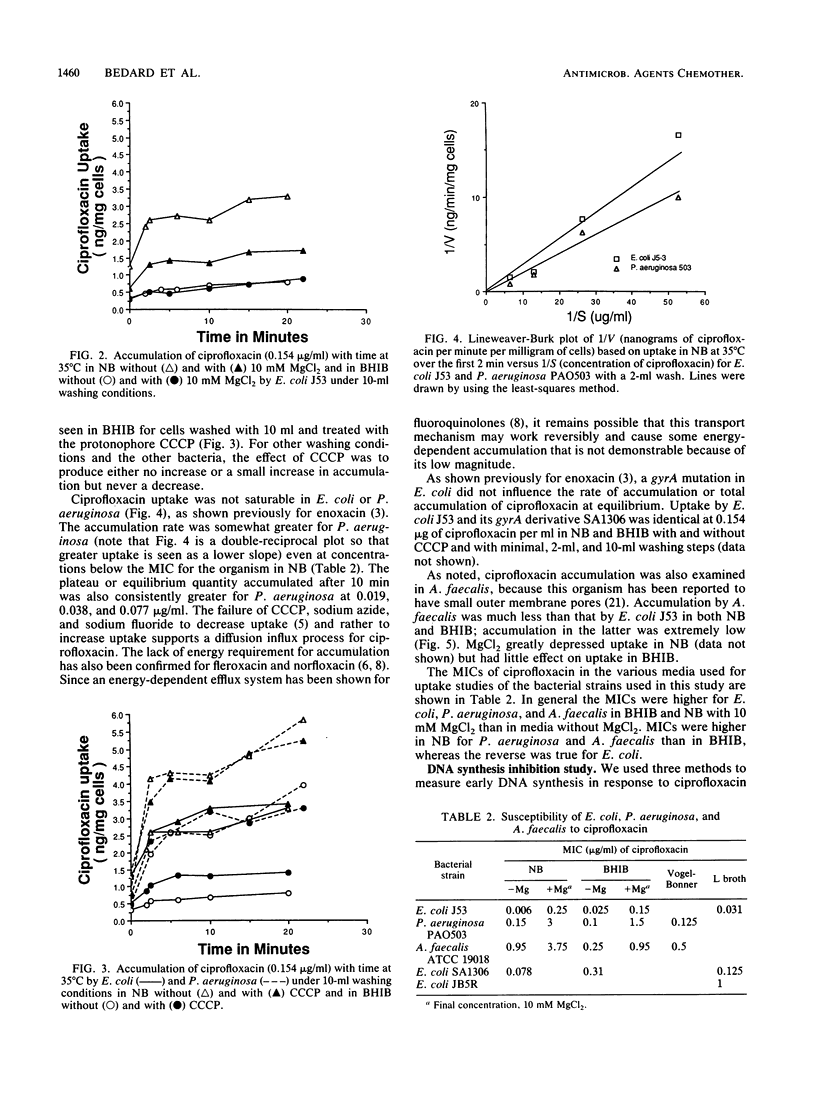
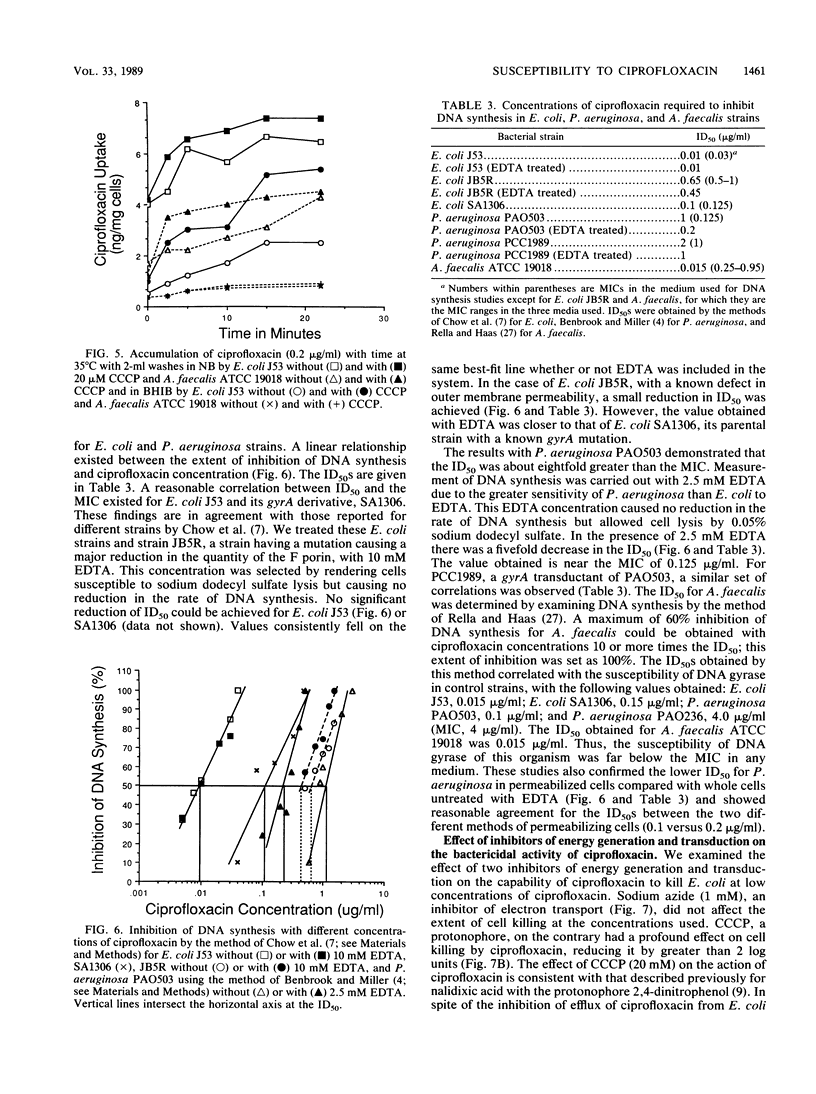
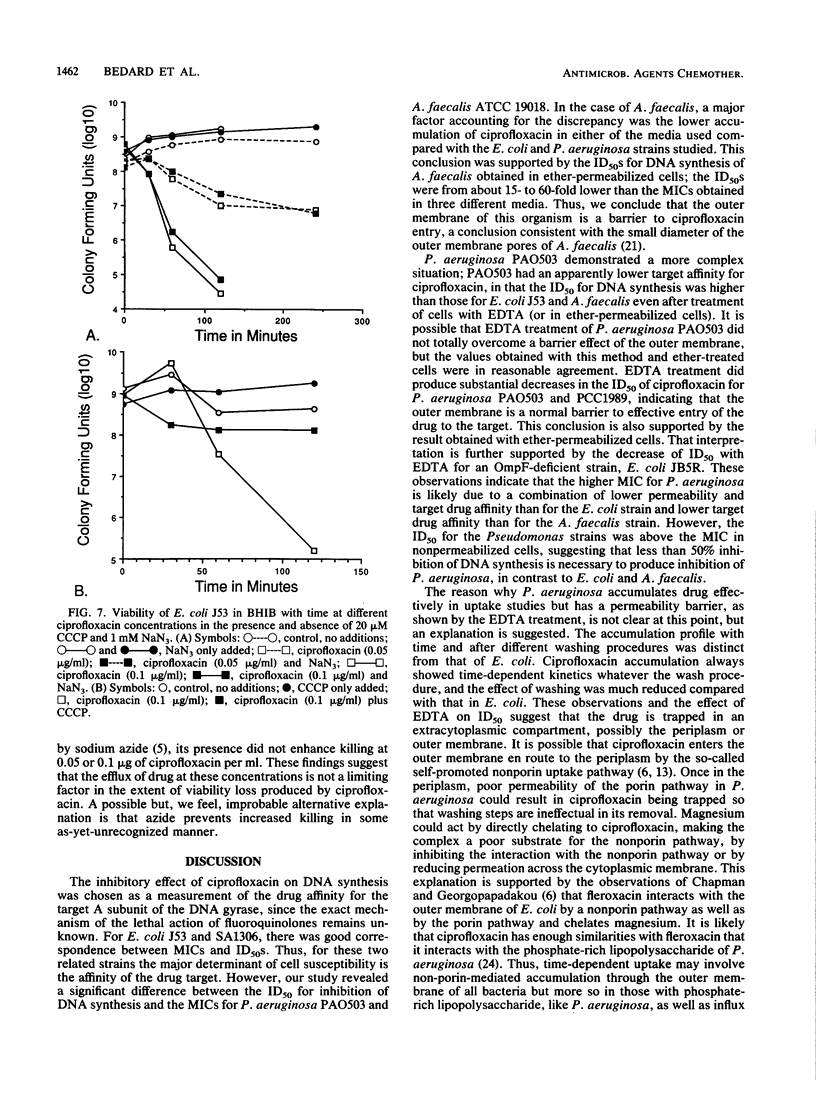
To examine the correlation between bacterial cell susceptibility to ciprofloxacin and the magnitude of uptake and cell target sensitivity, the relative contribution of ciprofloxacin accumulation in intact cells and its ability to inhibit DNA synthesis were investigated among strains of Escherichia coli, Pseudomonas aeruginosa, and Alcaligenes faecalis. Uptake studies of [14C]ciprofloxacin demonstrated diffusion kinetics for P. aeruginosa and E. coli. Ciprofloxacin was more readily removed from E. coli J53 and A. faecalis ATCC 19018 by washing than from P. aeruginosa PAO503. These results indicate that the process of cell accumulation is different for P. aeruginosa in that the drug is firmly bound at an extracellular site. Whatever the washing conditions, A. faecalis accumulated less drug than either of the other two bacteria. Magnesium chloride (10 mM) caused a substantial decrease of ciprofloxacin accumulated and an increase in the MIC, depending upon the nature of the medium. The addition of carbonyl cyanide m-chlorophenylhydrazone caused a variable increase in drug accumulated, depending on the medium and the bacterial strain. The concentration of ciprofloxacin required to obtain 50% inhibition (ID50) of DNA synthesis for P. aeruginosa PAO503 and A. faecalis ATCC 19018 did not correlate with their corresponding MICs but did for E. coli J53. Treatment with EDTA decreased the ID50 of ciprofloxacin for P. aeruginosa PAO503 and its gyrA derivative by 5- and 2-fold, respectively, and decreased the ID50 for E. coli JB5R, a strain with a known decrease in OmpF, by 1.4-fold but did not decrease the ID50 for the normally susceptible E. coli J53. The ID(50) for P. aeruginosa obtained after EDTA treatment or in ether-permeabilized cells was higher than that obtained for the other two strains. The protonophore carbonyl cyanide m-chlorophenylhydrazone prevented killing by low ciprofloxacin concentrtaions, but sodium azide did not. The latter compound did not enhance killing in association with inhibition of a previously described energy-dependent efflux of ciprofloxacin susceptibility being the susceptibility to inhibition of DNA synthesis in E. coli, poor premeability associated with the small pore size of A. faecalis, and a combination of low permeability and reduced susceptibility of DNA synthesis to inhibition for P. aeruginosa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama H., Fujimaki K., Sato K., Fujii T., Inoue M., Hirai K., Mitsuhashi S. Clinical isolate of Citrobacter freundii highly resistant to new quinolones. Antimicrob Agents Chemother. 1988 Jun;32(6):922–924. doi: 10.1128/aac.32.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama H., Sato K., Kato T., Hirai K., Mitsuhashi S. Norfloxacin resistance in a clinical isolate of Escherichia coli. Antimicrob Agents Chemother. 1987 Oct;31(10):1640–1641. doi: 10.1128/aac.31.10.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard J., Bryan L. E. Interaction of the fluoroquinolone antimicrobial agents ciprofloxacin and enoxacin with liposomes. Antimicrob Agents Chemother. 1989 Aug;33(8):1379–1382. doi: 10.1128/aac.33.8.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard J., Wong S., Bryan L. E. Accumulation of enoxacin by Escherichia coli and Bacillus subtilis. Antimicrob Agents Chemother. 1987 Sep;31(9):1348–1354. doi: 10.1128/aac.31.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook D. M., Miller R. V. Effects of norfloxacin on DNA metabolism in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1986 Jan;29(1):1–6. doi: 10.1128/aac.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Bedard J., Wong S., Chamberland S. Quinolone antimicrobial agents: mechanism of action and resistance development. Clin Invest Med. 1989 Feb;12(1):14–19. [PubMed] [Google Scholar]
- Chow R. T., Dougherty T. J., Fraimow H. S., Bellin E. Y., Miller M. H. Association between early inhibition of DNA synthesis and the MICs and MBCs of carboxyquinolone antimicrobial agents for wild-type and mutant [gyrA nfxB(ompF) acrA] Escherichia coli K-12. Antimicrob Agents Chemother. 1988 Aug;32(8):1113–1118. doi: 10.1128/aac.32.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., Hooper D. C., Wolfson J. S., Souza K. S., McMurry L. M., Levy S. B. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1187–1191. doi: 10.1128/aac.32.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook T. M., Deitz W. H., Goss W. A. Mechanism of action of nalidixic acid on Escherichia coli. IV. Effects on the stability of cellular constituents. J Bacteriol. 1966 Feb;91(2):774–779. doi: 10.1128/jb.91.2.774-779.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagala J. M., Hanna L. D., Heifetz C. L., Hutt M. P., Mich T. F., Sanchez J. P., Solomon M. New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. J Med Chem. 1986 Mar;29(3):394–404. doi: 10.1021/jm00153a015. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Moreau N., Kitzis M. D., Collatz E., Acar J. F., Goldstein F. W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985 Mar;151(3):501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Raffle V. J., Nicas T. I. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981 May;19(5):777–785. doi: 10.1128/aac.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Apr;31(4):582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Ng E. Y., Swartz M. N. Mechanisms of action of and resistance to ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):12–20. [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Ng E. Y., McHugh G. L., Swartz M. N. Mechanisms of quinolone resistance in Escherichia coli: characterization of nfxB and cfxB, two mutant resistance loci decreasing norfloxacin accumulation. Antimicrob Agents Chemother. 1989 Mar;33(3):283–290. doi: 10.1128/aac.33.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Sato K., Fujii T., Hirai K., Inoue M., Iyobe S., Mitsuhashi S. Some properties of subunits of DNA gyrase from Pseudomonas aeruginosa PAO1 and its nalidixic acid-resistant mutant. J Bacteriol. 1987 May;169(5):2322–2325. doi: 10.1128/jb.169.5.2322-2325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii J., Nakae T. Size of diffusion pore of Alcaligenes faecalis. Antimicrob Agents Chemother. 1988 Mar;32(3):378–384. doi: 10.1128/aac.32.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopman G., Macina O. T., Levinson M. E., Rosenkranz H. S. Computer automated structure evaluation of quinolone antibacterial agents. Antimicrob Agents Chemother. 1987 Nov;31(11):1831–1840. doi: 10.1128/aac.31.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin C. S., Smith J. T. Bactericidal mechanisms of ofloxacin. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):1–8. doi: 10.1093/jac/22.supplement_c.1. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J., Wijnands W. J., Wise R. Quinolone/ureidopenicillin cross-resistance. Lancet. 1987 Oct 17;2(8564):907–907. doi: 10.1016/s0140-6736(87)91387-0. [DOI] [PubMed] [Google Scholar]
- Rella M., Haas D. Resistance of Pseudomonas aeruginosa PAO to nalidixic acid and low levels of beta-lactam antibiotics: mapping of chromosomal genes. Antimicrob Agents Chemother. 1982 Aug;22(2):242–249. doi: 10.1128/aac.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J., Scarpa A. L. Genetic and physiological characterization of ciprofloxacin resistance in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1988 Apr;32(4):535–539. doi: 10.1128/aac.32.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Watanakunakorn C. Emergence of resistance to beta-lactams, aminoglycosides, and quinolones during combination therapy for infection due to Serratia marcescens. J Infect Dis. 1986 Mar;153(3):617–619. doi: 10.1093/infdis/153.3.617. [DOI] [PubMed] [Google Scholar]
- Sato K., Inoue Y., Fujii T., Aoyama H., Inoue M., Mitsuhashi S. Purification and properties of DNA gyrase from a fluoroquinolone-resistant strain of Escherichia coli. Antimicrob Agents Chemother. 1986 Nov;30(5):777–780. doi: 10.1128/aac.30.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. L., Pernet A. G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: the target of the drugs is DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):307–311. doi: 10.1073/pnas.82.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata M., Nishino T. DNA gyrase of Staphylococcus aureus and inhibitory effect of quinolones on its activity. Antimicrob Agents Chemother. 1988 Aug;32(8):1192–1195. doi: 10.1128/aac.32.8.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornaletti S., Pedrini A. M. Studies on the interaction of 4-quinolones with DNA by DNA unwinding experiments. Biochim Biophys Acta. 1988 Mar 31;949(3):279–287. doi: 10.1016/0167-4781(88)90153-4. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]
- Woodruff W. A., Parr T. R., Jr, Hancock R. E., Hanne L. F., Nicas T. I., Iglewski B. H. Expression in Escherichia coli and function of Pseudomonas aeruginosa outer membrane porin protein F. J Bacteriol. 1986 Aug;167(2):473–479. doi: 10.1128/jb.167.2.473-479.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama H., Nakae T. A small diffusion pore in the outer membrane of Pseudomonas aeruginosa. Eur J Biochem. 1986 May 15;157(1):33–38. doi: 10.1111/j.1432-1033.1986.tb09634.x. [DOI] [PubMed] [Google Scholar]
- Zweerink M. M., Edison A. Inhibition of Micrococcus luteus DNA gyrase by norfloxacin and 10 other quinolone carboxylic acids. Antimicrob Agents Chemother. 1986 Apr;29(4):598–601. doi: 10.1128/aac.29.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]


