Abstract
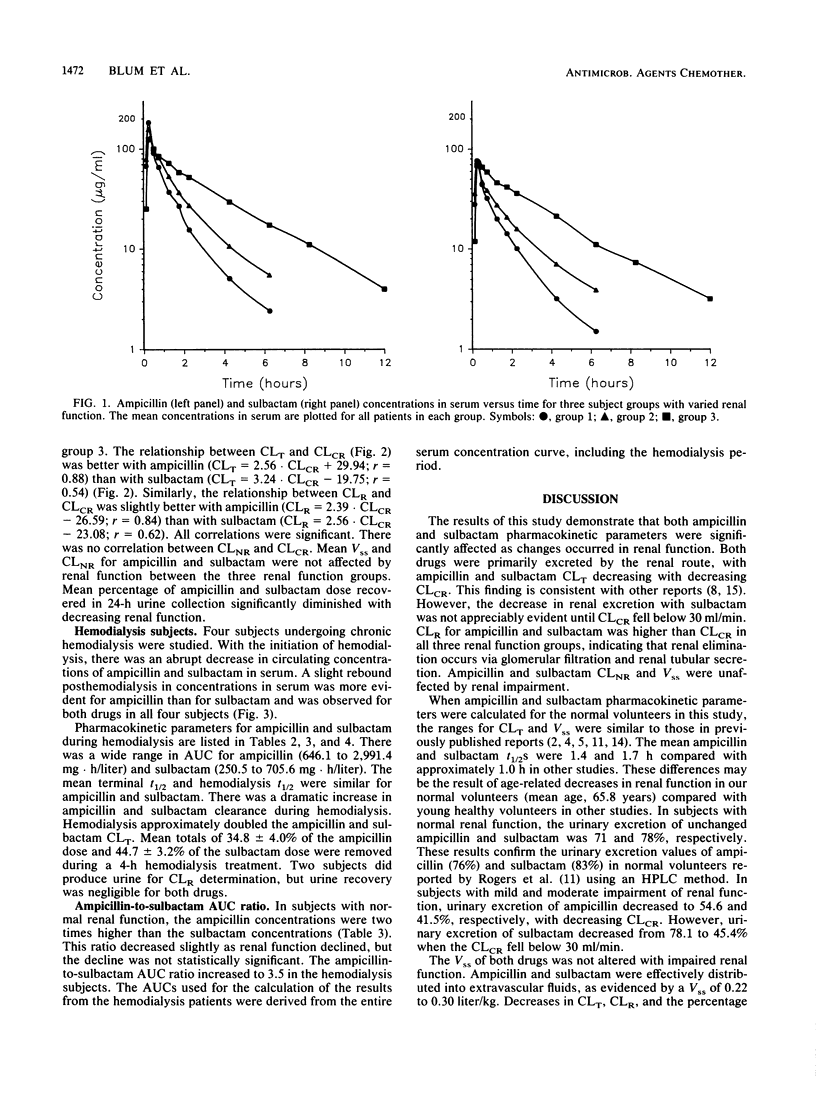
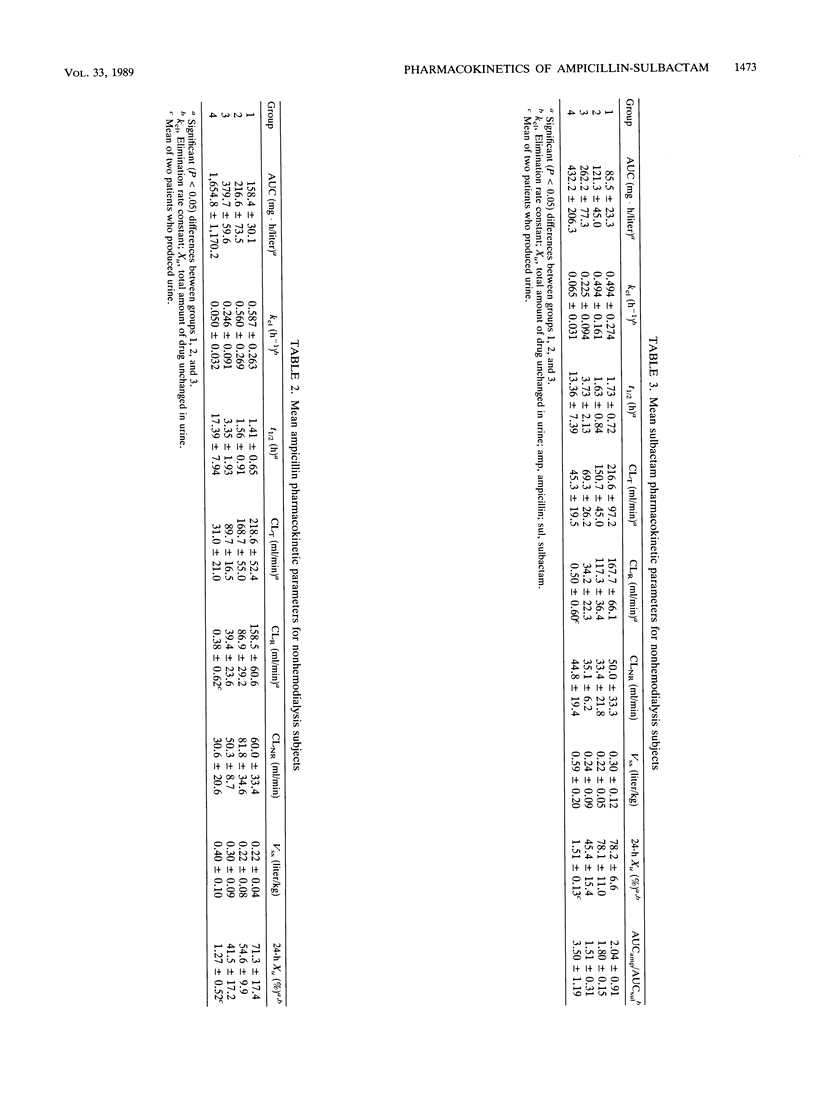
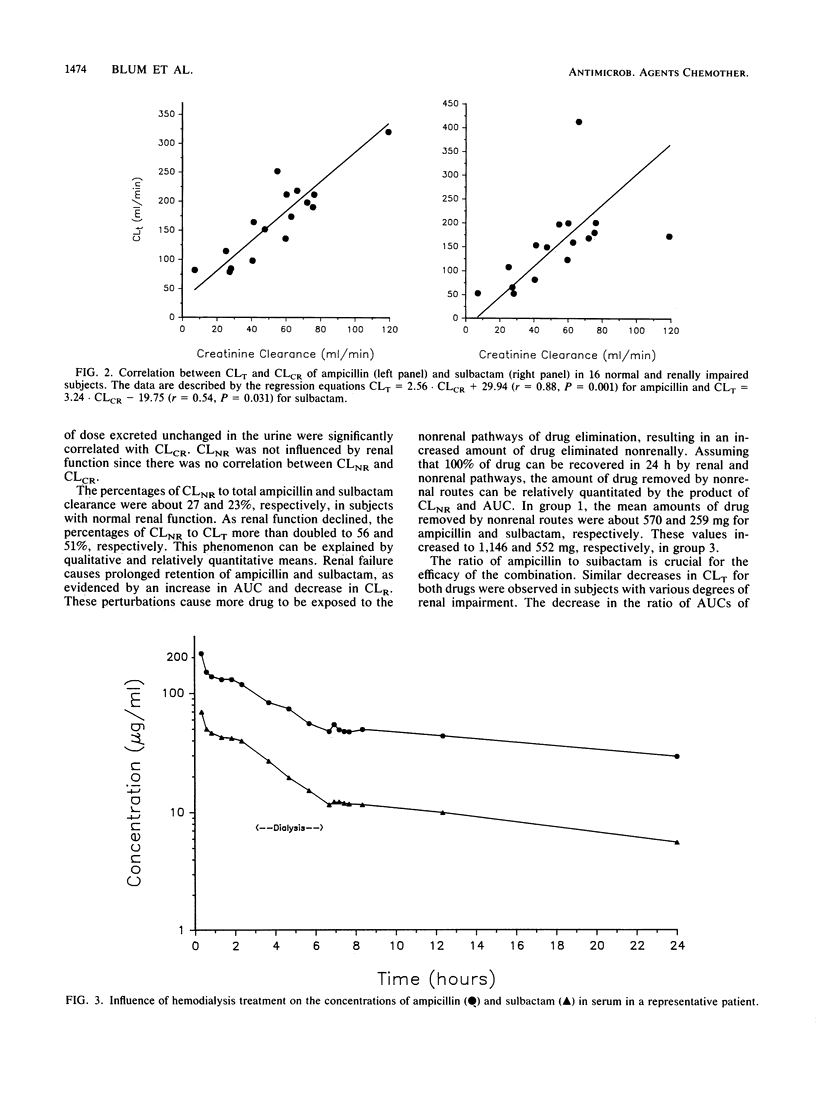
The single-dose pharmacokinetics of intravenously administered ampicillin (2.0 g) and sulbactam (1.0 g) were studied in normal subjects and in patients with various degrees of creatinine clearance (CLCR). Six normal subjects (CLCR, greater than 60 ml/min), six patients with mild renal failure (CLCR, 31 to 60 ml/min), four patients with severe renal failure (CLCR, 7 to 30 ml/min), and four patients requiring maintenance hemodialysis (CLCR, less than 7 ml/min) were studied. The terminal half-lives for ampicillin and sulbactam more than doubled in patients with severe renal failure compared with subjects with normal renal function and mild renal insufficiency. CLCR significantly correlated with ampicillin (r = 0.88) and sulbactam (r = 0.54) total body clearance. Mean steady-state volume of distribution and nonrenal clearance for ampicillin and sulbactam were not affected by renal function. Hemodialysis approximately doubled the ampicillin and sulbactam total body clearance. Mean totals of 34.8 +/- 4.0% of the ampicillin dose and 44.7 +/- 3.2% of the sulbactam dose were removed during a 4-h hemodialysis treatment. A slight rebound in concentrations in serum after hemodialysis was observed for both drugs in all four subjects. In hemodialysis patients, the ampicillin half-life was 17.4 +/- 8.0 h and the sulbactam half-life was 13.4 +/- 7.4 h. The ampicillin and sulbactam half-lives were appreciably altered during the hemodialysis period (means of 2.2 and 2.3 h, respectively). The nearly parallel decrease in total body clearance, with volume of distribution and nonrenal clearance remaining relatively constant, suggests that the same ratio of ampicillin to sulbactam is appropriate regardless of renal function. An adjustment of the ampicillin (2.0 g) and sulbactam (1.0 g) dose to twice daily would be appropriate in patients with a CLCR between 7 and 30 ml/min. Doses should be given every 24 h for those undergoing maintenance hemodialysis. On hemodialysis days, doses should be given after hemodialysis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bawdon R. E., Madsen P. O. High-pressure liquid chromatographic assay of sulbactam in plasma, urine, and tissue. Antimicrob Agents Chemother. 1986 Aug;30(2):231–233. doi: 10.1128/aac.30.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. M., Wise R., Andrews J. M., Hancox J. Comparative pharmacokinetics and tissue penetration of sulbactam and ampicillin after concurrent intravenous administration. Antimicrob Agents Chemother. 1982 Apr;21(4):565–567. doi: 10.1128/aac.21.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English A. R., Retsema J. A., Girard A. E., Lynch J. E., Barth W. E. CP-45,899, a beta-lactamase inhibitor that extends the antibacterial spectrum of beta-lactams: initial bacteriological characterization. Antimicrob Agents Chemother. 1978 Sep;14(3):414–419. doi: 10.1128/aac.14.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds G., Knirsch A. K., Stankewich J. P., Weidler D. R. The parenteral kinetics of ampicillin/sulbactam in man. Int J Clin Pharmacol Res. 1985;5(2):79–86. [PubMed] [Google Scholar]
- Foulds G., Stankewich J. P., Marshall D. C., O'Brien M. M., Hayes S. L., Weidler D. J., McMahon F. G. Pharmacokinetics of sulbactam in humans. Antimicrob Agents Chemother. 1983 May;23(5):692–699. doi: 10.1128/aac.23.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson T. P., Matusik E., Nelson L. D., Briggs W. A. Artificial kidneys and clearance calculations. Clin Pharmacol Ther. 1976 Dec;20(6):720–726. doi: 10.1002/cpt1976206720. [DOI] [PubMed] [Google Scholar]
- Jusko W. J., Lewis G. P., Schmitt G. W. Ampicillin and hetacillin pharmacokinetics in normal and anephric subjects. Clin Pharmacol Ther. 1973 Jan-Feb;14(1):90–99. doi: 10.1002/cpt197314190. [DOI] [PubMed] [Google Scholar]
- ROgers H. J., Bradbrook I. D., Morrison P. J., Spector R. G., Cox D. A., Lees L. J. Pharmacokinetics and bioavailability of sultamicillin estimated by high performance liquid chromatography. J Antimicrob Chemother. 1983 May;11(5):435–445. doi: 10.1093/jac/11.5.435. [DOI] [PubMed] [Google Scholar]
- Retsema J. A., English A. R., Girard A., Lynch J. E., Anderson M., Brennan L., Cimochowski C., Faiella J., Norcia W., Sawyer P. Sulbactam/ampicillin: in vitro spectrum, potency, and activity in models of acute infection. Rev Infect Dis. 1986 Nov-Dec;8 (Suppl 5):S528–S534. doi: 10.1093/clinids/8.supplement_5.s528. [DOI] [PubMed] [Google Scholar]
- Stec G. P., Atkinson A. J., Jr, Nevin M. J., Thenot J. P., Ruo T. I., Gibson T. P., Ivanovich P., del Greco F. N-Acetylprocainamide pharmacokinetics in functionally anephric patients before and after perturbation by hemodialysis. Clin Pharmacol Ther. 1979 Nov;26(5):618–628. doi: 10.1002/cpt1979265618. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Bedford K. A. Clavulanic acid and CP-45,899: a comparison of their in vitro activity in combination with penicillins. J Antimicrob Chemother. 1980 Mar;6(2):197–206. doi: 10.1093/jac/6.2.197. [DOI] [PubMed] [Google Scholar]
- Wise R., Donovan I. A., Andrews J. M., Drumm J., Bennett S. Penetration of sulbactam and ampicillin into peritoneal fluid. Antimicrob Agents Chemother. 1983 Aug;24(2):290–292. doi: 10.1128/aac.24.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N., Wise R. The elimination of sulbactam alone and combined with ampicillin in patients with renal dysfunction. J Antimicrob Chemother. 1983 Jun;11(6):583–587. doi: 10.1093/jac/11.6.583. [DOI] [PubMed] [Google Scholar]
- Ziemniak J. A., Cersosimo R. J., Russo J., Jr, Moran D. M., Kablitz C., Schentag J. J. Rebound following hemodialysis of cimetidine and its metabolites. Am J Kidney Dis. 1984 May;3(6):430–435. doi: 10.1016/s0272-6386(84)80006-2. [DOI] [PubMed] [Google Scholar]


