Abstract
Background—Human chorionic gonadotropin (hCG) is normally produced and secreted by trophoblastic cells during pregnancy and from gestational trophoblastic neoplasms. It is also detected in ovarian, stomach, and colon adenocarcinomas, as well as in squamous cell carcinoma of the oesophagus. Recently, interest in its role in the pathogenesis of tumours has been enlivened after the presence of βhCG in the cell membrane of several malignant cells was shown in vitro. Aims—To investigate the circulating concentrations of βhCG in patients with exocrine pancreatic adenocarcinoma and to examine its potential prognostic value. Patients—Thirty six patients with exocrine pancreatic adenocarcinoma, 12 patients with chronic pancreatitis, and 21 healthy volunteers were studied. Methods—βhCG serum concentrations were detected by the application of a radioimmunoassay technique. Results—Fifteen of 36 patients with pancreatic adenocarcinoma and only one patient with chronic pancreatitis had detectable plasma concentrations of βhCG (p<0.01). The patients with circulating serum titres of βhCG had a worse outcome compared with the group of βhCG negative patients: the difference was statistically significant (p=0.01). Conclusion—More than 40% of pancreatic exocrine tumours produce βhCG and its production is correlated with an adverse effect on outcome.
Keywords: β-human chorionic gonadotropin; chorionic gonadotropin; pancreatic cancer; tumour marker; paraneoplastic syndrome
Full Text
The Full Text of this article is available as a PDF (115.5 KB).
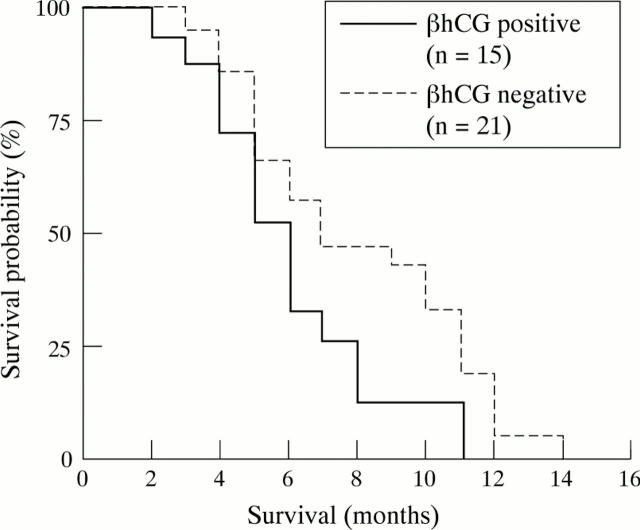
Figure 1 .
Survival curves for patients with pancreatic adenocarcinoma relative to βhCG status (p<0.01).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acevedo H. F., Kellen J. A., Wong A. C., Gardner H. A., Szalai J. P. Expression of human choriogonadotropin-like material correlates with metastatic phenotype of R3230 AC rat adenocarcinoma. Cancer Invest. 1987;5(3):177–185. doi: 10.3109/07357908709011734. [DOI] [PubMed] [Google Scholar]
- Acevedo H. F., Krichevsky A., Campbell-Acevedo E. A., Galyon J. C., Buffo M. J., Hartsock R. J. Expression of membrane-associated human chorionic gonadotropin, its subunits, and fragments by cultured human cancer cells. Cancer. 1992 Apr 1;69(7):1829–1842. doi: 10.1002/1097-0142(19920401)69:7<1829::aid-cncr2820690727>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Acevedo H. F., Tong J. Y., Hartsock R. J. Human chorionic gonadotropin-beta subunit gene expression in cultured human fetal and cancer cells of different types and origins. Cancer. 1995 Oct 15;76(8):1467–1475. doi: 10.1002/1097-0142(19951015)76:8<1467::aid-cncr2820760826>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Adcock E. W., 3rd, Teasdale T., August C. S., Cox S., Meschia G., Ballaglia T. C., Naughton M. A. Human chorionic gonadotropin: its possible role in maternal lymphocyte suppression. Science. 1973 Aug 31;181(4102):845–847. doi: 10.1126/science.181.4102.845. [DOI] [PubMed] [Google Scholar]
- Bagshawe K. D. Choriocarcinoma. A model for tumour markers. Acta Oncol. 1992;31(1):99–106. doi: 10.3109/02841869209088275. [DOI] [PubMed] [Google Scholar]
- Birkenfeld S., Noiman G., Krispin M., Schwartz S., Zakut H. The incidence and significance of serum hCG and CEA in patients with gastrointestinal malignant tumors. Eur J Surg Oncol. 1989 Apr;15(2):103–108. [PubMed] [Google Scholar]
- Braunstein G. D., Vaitukaitis J. L., Carbone P. P., Ross G. T. Ectopic production of human chorionic gonadotrophin by neoplasms. Ann Intern Med. 1973 Jan;78(1):39–45. doi: 10.7326/0003-4819-78-1-39. [DOI] [PubMed] [Google Scholar]
- Buckley C. H., Fox H. An immunohistochemical study of the significance of HCG secretion by large bowel adenocarcinomata. J Clin Pathol. 1979 Apr;32(4):368–372. doi: 10.1136/jcp.32.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. G., Iles R. K., Neven P., Ind T. E., Shepherd J. H., Chard T. Measurement of urinary beta core fragment of human chorionic gonadotrophin in women with vulvovaginal malignancy and its prognostic significance. Br J Cancer. 1995 Feb;71(2):350–353. doi: 10.1038/bjc.1995.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMagno E. P. Early diagnosis of chronic pancreatitis and pancreatic cancer. Med Clin North Am. 1988 Sep;72(5):979–992. doi: 10.1016/s0025-7125(16)30725-8. [DOI] [PubMed] [Google Scholar]
- Gailani S., Chu T. M., Nussbaum A., Ostrander M., Christoff N. Human chorionic gonadotrophins (hCG) in non-trophoblastic neoplasms. Assessment of abnormalities of hCG and CEA in bronchogenic and digestive neoplasms. Cancer. 1976 Oct;38(4):1684–1686. doi: 10.1002/1097-0142(197610)38:4<1684::aid-cncr2820380440>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Greenway B., Iqbal M. J., Johnson P. J., Williams R. Low serum testosterone concentrations in patients with carcinoma of the pancreas. Br Med J (Clin Res Ed) 1983 Jan 8;286(6359):93–95. doi: 10.1136/bmj.286.6359.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurchot C. The trophoblast theory of cancer (John Beard, 1857-1924) revisited. Oncology. 1975;31(5-6):310–333. doi: 10.1159/000225037. [DOI] [PubMed] [Google Scholar]
- Hattori M., Fukase M., Yoshimi H., Matsukura S., Imura H. Ectopic production of human chorionic gonadotropin in malignant tumors. Cancer. 1978 Nov;42(5):2328–2333. doi: 10.1002/1097-0142(197811)42:5<2328::aid-cncr2820420533>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Klöppel G., Maillet B. Classification and staging of pancreatic nonendocrine tumors. Radiol Clin North Am. 1989 Jan;27(1):105–119. [PubMed] [Google Scholar]
- Melmed S., Braunstein G. D. Human chorionic gonadotropin stimulates proliferation of Nb 2 rat lymphoma cells. J Clin Endocrinol Metab. 1983 May;56(5):1068–1070. doi: 10.1210/jcem-56-5-1068. [DOI] [PubMed] [Google Scholar]
- Metz K. A., Richter H. J., Leder L. D. Adenocarcinoma of the colon with syncytiotrophoblastic differentiation: differential diagnosis and implications. Pathol Res Pract. 1985 Jan;179(3):419–424. doi: 10.1016/s0344-0338(85)80152-7. [DOI] [PubMed] [Google Scholar]
- Moossa A. R., Levin B. The diagnosis of "early" pancreatic cancer: the University of Chicago experience. Cancer. 1981 Mar 15;47(6 Suppl):1688–1697. doi: 10.1002/1097-0142(19810315)47:6+<1688::aid-cncr2820471438>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Muggia F. M., Rosen S. W., Weintraub B. D., Hansen H. H. Ectopic placental proteins in nontrophoblastic tumors. Serial measurements following chemotherapy. Cancer. 1975 Oct;36(4):1327–1337. doi: 10.1002/1097-0142(197510)36:4<1327::aid-cncr2820360421>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Nix G. A., Schmitz P. I., Wilson J. H., Van Blankenstein M., Groeneveld C. F., Hofwijk R. Carcinoma of the head of the pancreas. Therapeutic implications of endoscopic retrograde cholangiopancreatography findings. Gastroenterology. 1984 Jul;87(1):37–43. [PubMed] [Google Scholar]
- Placental proteins and their subunits as tumor markers. Ann Intern Med. 1975 Jan;82(1):71–83. doi: 10.7326/0003-4819-82-1-71. [DOI] [PubMed] [Google Scholar]
- Regelson W. Have we found the "definitive cancer biomarker"? The diagnostic and therapeutic implications of human chorionic gonadotropin-beta expression as a key to malignancy. Cancer. 1995 Oct 15;76(8):1299–1301. doi: 10.1002/1097-0142(19951015)76:8<1299::aid-cncr2820760802>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Rosen S. W., Weintraub B. D., Aaronson S. A. Nonrandom ectopic protein production by malignant cells: direct evidence in vitro. J Clin Endocrinol Metab. 1980 May;50(5):834–841. doi: 10.1210/jcem-50-5-834. [DOI] [PubMed] [Google Scholar]
- Savarino V., Mansi C., Bistolfi L., Zentilin P., Celle G. Failure of new diagnostic aids in improving detection of pancreatic cancer at a resectable stage. Dig Dis Sci. 1983 Dec;28(12):1078–1082. doi: 10.1007/BF01295805. [DOI] [PubMed] [Google Scholar]
- Shousha S., Chappell R., Matthews J., Cooke T. Human chorionic gonadotrophin expression in colorectal adenocarcinoma. Dis Colon Rectum. 1986 Sep;29(9):558–560. doi: 10.1007/BF02554253. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Weintraub B. D., Barowsky N. J., Rabson A. S., Rosen S. W. Subunits of human chorionic gonadotropin: unbalanced synthesis and secretion by clonal cell strains derived from a bronchogenic carcinoma. Proc Natl Acad Sci U S A. 1973 May;70(5):1419–1422. doi: 10.1073/pnas.70.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L., Ross G. T., Braunstein G. D., Rayford P. L. Gonadotropins and their subunits: basic and clinical studies. Recent Prog Horm Res. 1976;32:289–331. doi: 10.1016/b978-0-12-571132-6.50019-1. [DOI] [PubMed] [Google Scholar]
- Van Rinsum J., Smets L. A., Van Rooy H., Van den Eijnden D. H. Specific inhibition of human natural killer cell-mediated cytotoxicity by sialic acid and sialo-oligosaccharides. Int J Cancer. 1986 Dec 15;38(6):915–922. doi: 10.1002/ijc.2910380620. [DOI] [PubMed] [Google Scholar]
- Wade T. P., Radford D. M., Virgo K. S., Johnson F. E. Complications and outcomes in the treatment of pancreatic adenocarcinoma in the United States veteran. J Am Coll Surg. 1994 Jul;179(1):38–48. [PubMed] [Google Scholar]
- Watanapa P., Williamson R. C. Surgical palliation for pancreatic cancer: developments during the past two decades. Br J Surg. 1992 Jan;79(1):8–20. doi: 10.1002/bjs.1800790105. [DOI] [PubMed] [Google Scholar]