Abstract
Background—Increased concentrations of 5-hydroxytryptamine (5-HT) can be detected in the systemic circulation after a meal and may be involved in the physiological control of gastrointestinal motility. Abnormalities of 5-HT release after a meal might explain some of the postprandial symptoms associated with the irritable bowel syndrome (IBS). Aim—To investigate the effect of a standard meal on plasma 5-HT and urinary 5-hydroxyindole acetic acid (5-HIAA) concentrations in patients with diarrhoea predominant IBS and in healthy volunteers. Methods—After an overnight fast, six volunteers and five patients with IBS were given a carbohydrate-rich meal. Blood and urine samples were taken before and for four hours after the meal. Platelet-poor plasma 5-HT and urinary 5-HIAA were analysed by reversed phase high performance liquid chromatography with fluorometric detection. 5-HIAA was expressed as a ratio with urinary creatinine concentration, which was measured by spectrophotometry. Results—During the four hour postprandial period, 5-HT concentrations were significantly higher in patients with IBS than in healthy volunteers at 0.5 hours (p<0.05), 2 hours (p<0.05) and 2.5 hours (p<0.05). 5-HT was not detected in the plasma in the fasting state in patients or volunteers. Median peak 5-HT in patients with IBS (359 (198-796) nmol/l) was significantly greater than volunteers (83 (7-190)) (p<0.05). "Area under the curve" for 5-HT detection was greater for patients with IBS (317(138-771)) than for healthy volunteers (51 (4-129); p<0.05).The duration of the 5-HT peak was significantly longer in patients with IBS (3 (1-3) hours) than in the healthy volunteers (1 (1-1) hours; p<0.01). Postprandial urinary median 5-HIAA values in controls (5.6 (5.5-5.8) µmol/mmol creatinine) and patients with IBS (3.0(2.5-6.8) µmol/mmol creatinine) were not significantly different from preprandial values (controls: 5.9 (5.5-6.6) µmol/mmol creatinine; patients with IBS: (6.2 (2.4-9.3) µmol/mmol creatinine). Conclusion—These findings indicate that there may be a difference in the way that 5-HT is released in patients with diarrhoea predominant IBS, and could suggest a possible role for 5-HT in the postprandial symptoms of these patients.
Keywords: 5-hydroxytryptamine; postprandial; diarrhoea predominant irritable bowel syndrome
Full Text
The Full Text of this article is available as a PDF (116.7 KB).
Figure 1 .
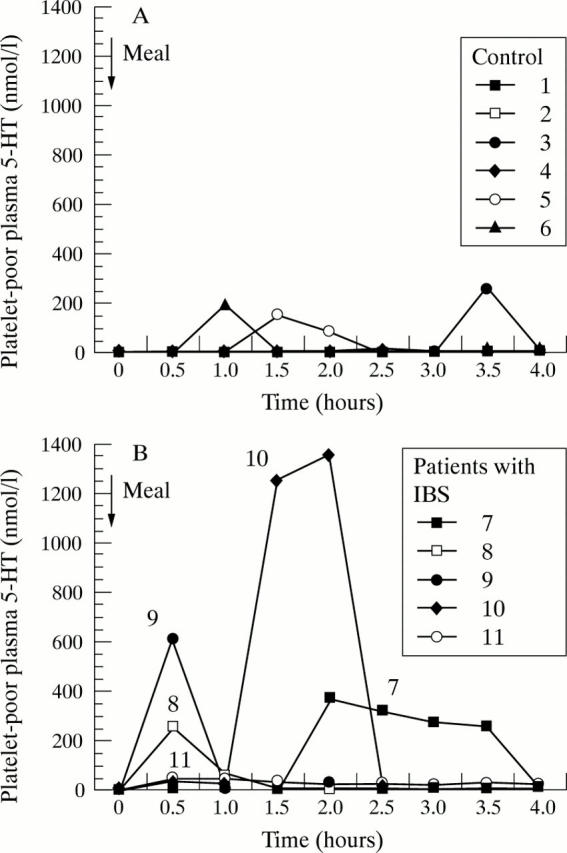
(A) Postprandial 5-HT profiles in patients with diarrhoea predominant IBS after a meal at zero hours. (B) Postprandial 5-HT profiles in healthy volunteers after a meal at zero hours.
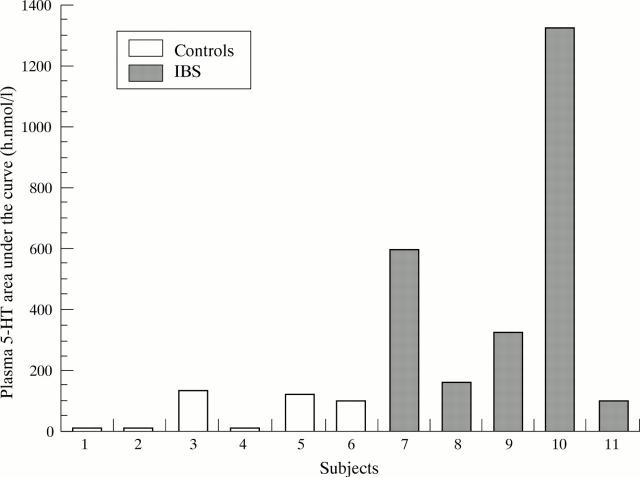
Figure 2 .
Plasma 5-HT "area under the curve" in patients with diarrhoea predominant IBS and healthy volunteers.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahonen A., Kyösola K., Penttilä O. Enterochromaffin cells in macrophages in ulcerative colitis and irritable colon. Ann Clin Res. 1976 Feb;8(1):1–7. [PubMed] [Google Scholar]
- Anderson G. M., Feibel F. C., Cohen D. J. Determination of serotonin in whole blood, platelet-rich plasma, platelet-poor plasma and plasma ultrafiltrate. Life Sci. 1987 Mar 16;40(11):1063–1070. doi: 10.1016/0024-3205(87)90568-6. [DOI] [PubMed] [Google Scholar]
- Anderson G. M., Feibel F. C., Wetlaufer L. A., Schlicht K. R., Ort S. M., Cohen D. J. Effect of a meal on human whole blood serotonin. Gastroenterology. 1985 Jan;88(1 Pt 1):86–89. doi: 10.1016/s0016-5085(85)80137-2. [DOI] [PubMed] [Google Scholar]
- Anderson J. V., Coupe M. O., Morris J. A., Hodgson H. J., Bloom S. R. Remission of symptoms in carcinoid syndrome with a new 5-hydroxytryptamine M receptor antagonist. Br Med J (Clin Res Ed) 1987 May 2;294(6580):1129–1129. doi: 10.1136/bmj.294.6580.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. L., Rapeport W. G., Sanger G. J. Neuropharmacology of emesis induced by anti-cancer therapy. Trends Pharmacol Sci. 1988 Sep;9(9):334–341. doi: 10.1016/0165-6147(88)90106-x. [DOI] [PubMed] [Google Scholar]
- Artigas F., Sarrias M. J., Martínez E., Gelpí E. Serotonin in body fluids: characterization of human plasmatic and cerebrospinal fluid pools by means of a new HPLC method. Life Sci. 1985 Aug 5;37(5):441–447. doi: 10.1016/0024-3205(85)90406-0. [DOI] [PubMed] [Google Scholar]
- BULBRING E., CREMA A. Observations concerning the action of 5-hydroxytryptamine on the peristaltic reflex. Br J Pharmacol Chemother. 1958 Dec;13(4):444–457. doi: 10.1111/j.1476-5381.1958.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badcock N. R., Spence J. G., Stern L. M. Blood serotonin levels in adults, autistic and non-autistic children--with a comparison of different methodologies. Ann Clin Biochem. 1987 Nov;24(Pt 6):625–634. doi: 10.1177/000456328702400613. [DOI] [PubMed] [Google Scholar]
- Barnes N. M., Ge J., Jones W. G., Naylor R. J., Rudd J. A. Cisplatin induced emesis: preliminary results indicative of changes in plasma levels of 5-hydroxytryptamine. Br J Cancer. 1990 Nov;62(5):862–864. doi: 10.1038/bjc.1990.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearcroft C. P., Farthing M. J., Perrett D. Determination of 5-hydroxytryptamine, 5-hydroxyindoleacetic acid and tryptophan in plasma and urine by HPLC with fluorimetric detection. Biomed Chromatogr. 1995 Jan-Feb;9(1):23–27. doi: 10.1002/bmc.1130090105. [DOI] [PubMed] [Google Scholar]
- Beck O., Wallén N. H., Bröijersén A., Larsson P. T., Hjemdahl P. On the accurate determination of serotonin in human plasma. Biochem Biophys Res Commun. 1993 Oct 15;196(1):260–266. doi: 10.1006/bbrc.1993.2243. [DOI] [PubMed] [Google Scholar]
- Bertaccini G. Tissue 5-hydroxytryptamine and urinary 5-hydroxyindoleacetic acid after partial or total removal of the gastro-intestinal tract in the rat. J Physiol. 1960 Sep;153(2):239–249. doi: 10.1113/jphysiol.1960.sp006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beubler E., Bukhave K., Rask-Madsen J. Significance of calcium for the prostaglandin E2-mediated secretory response to 5-hydroxytryptamine in the small intestine of the rat in vivo. Gastroenterology. 1986 Jun;90(6):1972–1977. doi: 10.1016/0016-5085(86)90269-6. [DOI] [PubMed] [Google Scholar]
- Blum I., Vered Y., Graff E., Grosskopf Y., Don R., Harsat A., Raz O. The influence of meal composition on plasma serotonin and norepinephrine concentrations. Metabolism. 1992 Feb;41(2):137–140. doi: 10.1016/0026-0495(92)90141-v. [DOI] [PubMed] [Google Scholar]
- CHAUDHARY N. A., TRUELOVE S. C. The irritable colon syndrome. A study of the clinical features, predisposing causes, and prognosis in 130 cases. Q J Med. 1962 Jul;31:307–322. [PubMed] [Google Scholar]
- Cann P. A., Read N. W., Brown C., Hobson N., Holdsworth C. D. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut. 1983 May;24(5):405–411. doi: 10.1136/gut.24.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook I. J., van Eeden A., Collins S. M. Patients with irritable bowel syndrome have greater pain tolerance than normal subjects. Gastroenterology. 1987 Oct;93(4):727–733. doi: 10.1016/0016-5085(87)90434-3. [DOI] [PubMed] [Google Scholar]
- Cubeddu L. X., Hoffmann I. S., Fuenmayor N. T., Finn A. L. Efficacy of ondansetron (GR 38032F) and the role of serotonin in cisplatin-induced nausea and vomiting. N Engl J Med. 1990 Mar 22;322(12):810–816. doi: 10.1056/NEJM199003223221204. [DOI] [PubMed] [Google Scholar]
- Cubeddu L. X., Hoffmann I. S., Fuenmayor N. T., Malave J. J. Changes in serotonin metabolism in cancer patients: its relationship to nausea and vomiting induced by chemotherapeutic drugs. Br J Cancer. 1992 Jul;66(1):198–203. doi: 10.1038/bjc.1992.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubeddu L. X., Hoffmann I. S. Participation of serotonin on early and delayed emesis induced by initial and subsequent cycles of cisplatinum-based chemotherapy: effects of antiemetics. J Clin Pharmacol. 1993 Aug;33(8):691–697. doi: 10.1002/j.1552-4604.1993.tb05608.x. [DOI] [PubMed] [Google Scholar]
- Da Prada M., Tranzer J. P., Pletscher A. Storage of 5-hydroxytryptamine in human blood platelets. Experientia. 1972 Nov 15;28(11):1328–1329. doi: 10.1007/BF01965326. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Asarkof N., Pike G. Calcium dependence of serotonin-induced changes in rabbit ileal electrolyte transport. J Clin Invest. 1980 Aug;66(2):341–352. doi: 10.1172/JCI109862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERSPAMER V., TESTINI A. Observations on the release and turnover rate of 5-hydroxytryptamine in the gastrointestinal tract. J Pharm Pharmacol. 1959 Oct;11:618–623. doi: 10.1111/j.2042-7158.1959.tb12603.x. [DOI] [PubMed] [Google Scholar]
- Feldman J. M. Carcinoid tumors and syndrome. Semin Oncol. 1987 Sep;14(3):237–246. [PubMed] [Google Scholar]
- Flachaire E., Beney C., Berthier A., Salandre J., Quincy C., Renaud B. Determination of reference values for serotonin concentration in platelets of healthy newborns, children, adults, and elderly subjects by HPLC with electrochemical detection. Clin Chem. 1990 Dec;36(12):2117–2120. [PubMed] [Google Scholar]
- Gomborone J., Dewsnap P., Libby G., Farthing M. Abnormal illness attitudes in patients with irritable bowel syndrome. J Psychosom Res. 1995 Feb;39(2):227–230. doi: 10.1016/0022-3999(94)00126-p. [DOI] [PubMed] [Google Scholar]
- Gorard D. A., Libby G. W., Farthing M. J. 5-Hydroxytryptamine and human small intestinal motility: effect of inhibiting 5-hydroxytryptamine reuptake. Gut. 1994 Apr;35(4):496–500. doi: 10.1136/gut.35.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorard D. A., Libby G. W., Farthing M. J. Ambulatory small intestinal motility in 'diarrhoea' predominant irritable bowel syndrome. Gut. 1994 Feb;35(2):203–210. doi: 10.1136/gut.35.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore S., Gilmore I. T., Haigh C. G., Brownless S. M., Stockdale H., Morris A. I. Colonic transit in man is slowed by ondansetron (GR38032F), a selective 5-hydroxytryptamine receptor (type 3) antagonist. Aliment Pharmacol Ther. 1990 Apr;4(2):139–144. doi: 10.1111/j.1365-2036.1990.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Kellum J. M., Jaffe B. M. Validation and application of a radioimmunoassay for serotonin. Gastroenterology. 1976 Apr;70(4):516–522. [PubMed] [Google Scholar]
- Manning A. P., Thompson W. G., Heaton K. W., Morris A. F. Towards positive diagnosis of the irritable bowel. Br Med J. 1978 Sep 2;2(6138):653–654. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuchansky C., Launay J. M. Serotonin, catecholamines, and spontaneous midgut carcinoid flush: plasma studies from flushing and nonflushing sites. Gastroenterology. 1995 Mar;108(3):743–751. doi: 10.1016/0016-5085(95)90447-6. [DOI] [PubMed] [Google Scholar]
- Märtensson H. G., Zinner M. J., Jaffe B. M. Effects of intraluminal perfusion with serotonin on jejunal motility. Dig Dis Sci. 1986 Aug;31(8):811–816. doi: 10.1007/BF01296048. [DOI] [PubMed] [Google Scholar]
- Palmer R. L., Stonehill E., Crisp A. H., Waller S. L., Misiewicz J. J. Psychological characteristics of patients with the irritable bowel syndrome. Postgrad Med J. 1974 Jul;50(585):416–419. doi: 10.1136/pgmj.50.585.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior A., Read N. W. Reduction of rectal sensitivity and post-prandial motility by granisetron, a 5 HT3-receptor antagonist, in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1993 Apr;7(2):175–180. doi: 10.1111/j.1365-2036.1993.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Scolapio J. S., Camilleri M., von der Ohe M. R., Hanson R. B. Ascending colon response to feeding: evidence for a 5-hydroxytryptamine-3 mechanism. Scand J Gastroenterol. 1995 Jun;30(6):562–567. doi: 10.3109/00365529509089790. [DOI] [PubMed] [Google Scholar]
- Steadman C. J., Talley N. J., Phillips S. F., Zinsmeister A. R. Selective 5-hydroxytryptamine type 3 receptor antagonism with ondansetron as treatment for diarrhea-predominant irritable bowel syndrome: a pilot study. Mayo Clin Proc. 1992 Aug;67(8):732–738. doi: 10.1016/s0025-6196(12)60797-6. [DOI] [PubMed] [Google Scholar]
- Tagari P. C., Boullin D. J., Davies C. L. Simplified determination of serotonin in plasma by liquid chromatography with electrochemical detection. Clin Chem. 1984 Jan;30(1):131–135. [PubMed] [Google Scholar]
- Talley N. J., Phillips S. F., Haddad A., Miller L. J., Twomey C., Zinsmeister A. R., MacCarty R. L., Ciociola A. GR 38032F (ondansetron), a selective 5HT3 receptor antagonist, slows colonic transit in healthy man. Dig Dis Sci. 1990 Apr;35(4):477–480. doi: 10.1007/BF01536922. [DOI] [PubMed] [Google Scholar]
- Vassallo M., Camilleri M., Phillips S. F., Brown M. L., Chapman N. J., Thomforde G. M. Transit through the proximal colon influences stool weight in the irritable bowel syndrome. Gastroenterology. 1992 Jan;102(1):102–108. doi: 10.1016/0016-5085(92)91789-7. [DOI] [PubMed] [Google Scholar]
- von der Ohe M. R., Camilleri M., Kvols L. K. A 5HT3 antagonist corrects the postprandial colonic hypertonic response in carcinoid diarrhea. Gastroenterology. 1994 May;106(5):1184–1189. doi: 10.1016/0016-5085(94)90008-6. [DOI] [PubMed] [Google Scholar]