Abstract
Background—Portal hypertension is associated with gross haemodynamic disturbances characterised by high cardiac output, low peripheral vascular resistance, increased splanchnic blood flow, and portal systemic shunting. Aims—To study the relationship between intrahepatic portal systemic shunts and microsphere induced portal hypertension in the rat liver. Methods—Different sized microspheres were sequentially injected into the portal vein of male Wistar rats. Results—Steady state portal venous pressure was increased by 102.2 (35.6)% (14.9 (3.6) mm Hg) and 272.3 (78.0)% (24.0 (2.2) mm Hg) above the basal pressure following sequential injections of 15 and 80 µm diameter microspheres, respectively. Sequential injection of 15, 40, and 80 µm diameter microspheres in either ascending or descending order of size did not generate further increases in portal venous pressure. A single injection of 1.8× 105 80 µm microspheres consistently produced a steady state portal venous pressure of 19.0 (1.3) mm Hg but did not approach the much higher value of 36.6 (43.2) mm Hg measured during clamping of the portal vein. These data indicate that the opening of patent intrahepatic shunts was responsible for the reduced pressures observed during microsphere injections and further evidence for this was provided by the location of microspheres in the pulmonary vascular bed. The elevation in portal venous pressure achieved by microsphere injections was not significantly different to that produced in rats subjected to partial portal vein ligation (20.7(0.5) mm Hg, p>0.05). Wedged hepatic venous pressure decreased from 6.7 (0.7) to 3.0 (0.6) mm Hg following injection of 80 µm microspheres, suggesting a decrease in total hepatic blood flow. Conversely, injection of 15 µm microspheres induced an increase in wedged hepatic venous pressure from 7.0 (1.0) mm Hg to 12.4(1.8) mm Hg, indicating a localised redistribution of blood flow at the presinusoidal level of the portal venous vascular network and increased intrahepatic shunt flow. Conclusion—It is suggested that there may be a protective pathophysiological role for these shunts when the liver is subjected to changes which induce acute portal hypertension.
Keywords: portal hypertension; intrahepatic shunts; rat liver; hepatic blood flow
Full Text
The Full Text of this article is available as a PDF (195.5 KB).
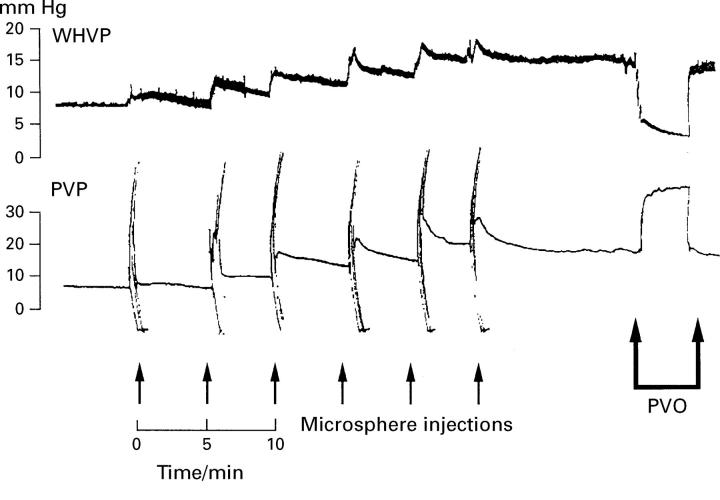
Figure 1 .
Portal venous pressure (PVP) and wedged hepatic venous pressure (WHVP) recordings from group 1 during intraportal injections of 15 µm diameter microspheres and subsequent portal vein occlusion (PVO).
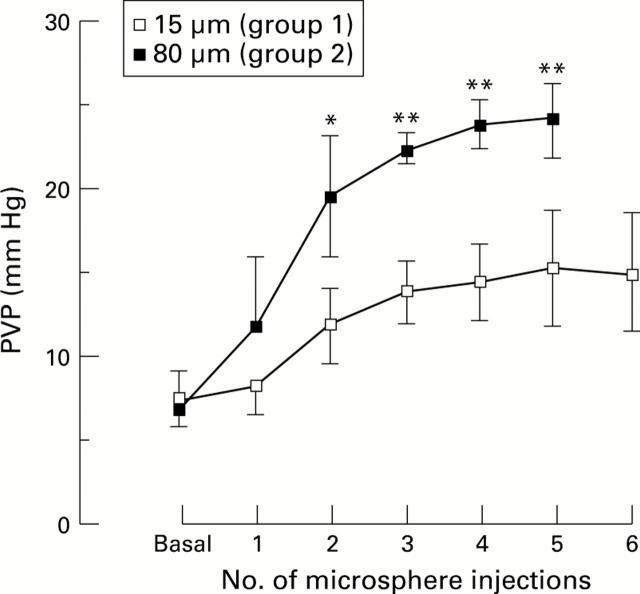
Figure 2 .
Changes in portal venous pressure (PVP) during intraportal microsphere injections. *p<0.05, **p<0.01, Student's unpaired t test, group 2 versus group 1.
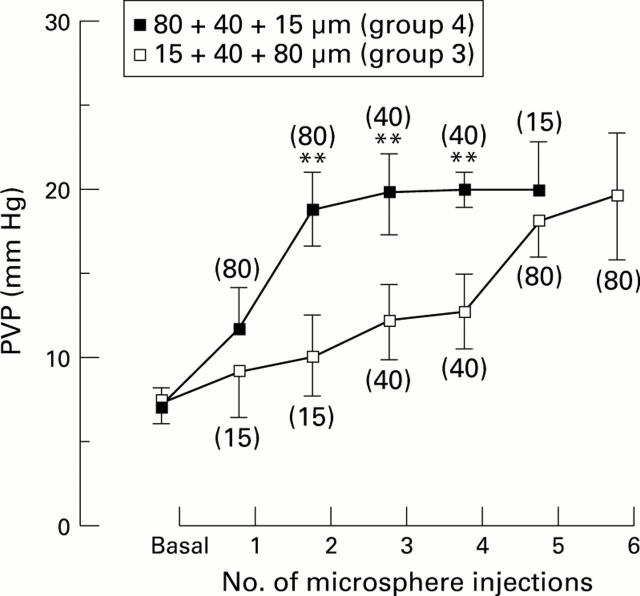
Figure 3 .
Changes in portal venous pressure (PVP) during intraportal microsphere injections. **p<0.01, Student's unpaired t test, group 4 versus group 3.
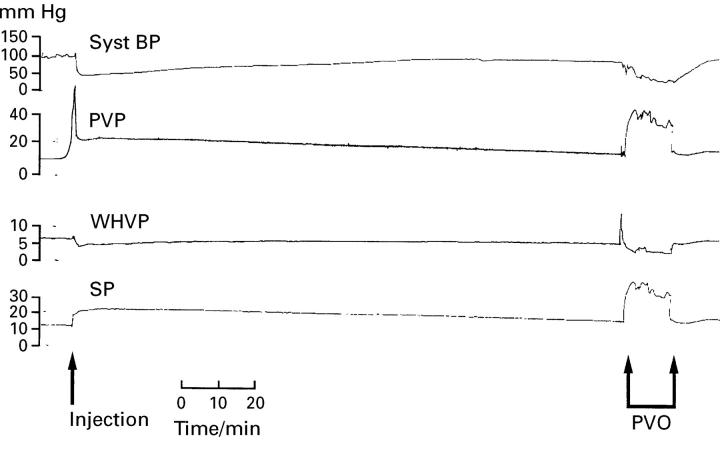
Figure 4 .
Systemic blood pressure (Syst BP), portal venous pressure (PVP), wedged hepatic venous pressure (WHVP), and splenic pulp pressure (SP) measurements after two aliquots of 80 µm diameter microspheres (group 5) were injected continuously into the portal vein. The portal venous pressure was elevated to a steady state value of 19.0 (1.3) mm Hg 150 minutes after injection. However, total occlusion of the portal vein at the liver hilus produced a further dramatic increase in portal venous pressure (PVO) which again suggested that not all the intrahepatic shunts had been occluded.
Figure 5 .
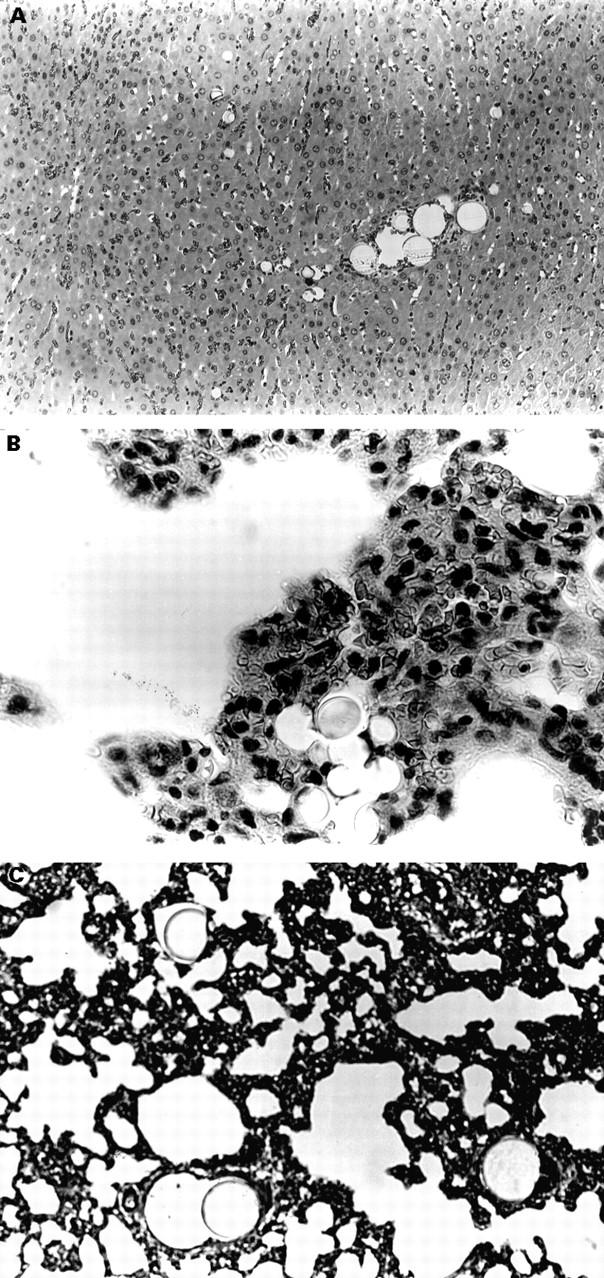
Histological sections showing (A) 15, 40, and 80 µm spheres lodged in liver (group 3, original magnification ×10), (B) 15 µm spheres lodged in lung (group 4, original magnification ×40), and (C) 80 µm spheres lodged in lung (group 2, original magnification ×16).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosch J., Mastai R., Kravetz D., Navasa M., Rodés J. Hemodynamic evaluation of the patient with portal hypertension. Semin Liver Dis. 1986 Nov;6(4):309–317. doi: 10.1055/s-2008-1040613. [DOI] [PubMed] [Google Scholar]
- Braillon A., Cailmail S., Gaudin C., Lebrec D. Reduced splanchnic vasoconstriction to angiotensin II in conscious rats with biliary cirrhosis. J Hepatol. 1993 Jan;17(1):86–90. doi: 10.1016/s0168-8278(05)80526-4. [DOI] [PubMed] [Google Scholar]
- Fernández-Muñoz D., Caramelo C., Santos J. C., Blanchart A., Hernando L., López-Novoa J. M. Systemic and splanchnic hemodynamic disturbances in conscious rats with experimental liver cirrhosis without ascites. Am J Physiol. 1985 Sep;249(3 Pt 1):G316–G320. doi: 10.1152/ajpgi.1985.249.3.G316. [DOI] [PubMed] [Google Scholar]
- Groszmann R. J., Glickman M., Blei A. T., Storer E., Conn H. O. Wedged and free hepatic venous pressure measured with a balloon catheter. Gastroenterology. 1979 Feb;76(2):253–258. [PubMed] [Google Scholar]
- Hoefs J. C., Reynolds T. B., Pare P., Sakimura I. A new method for the measurement of intrahepatic shunts. J Lab Clin Med. 1984 Mar;103(3):446–461. [PubMed] [Google Scholar]
- Huet P. M., Pomier-Layrargues G., Villeneuve J. P., Varin F., Viallet A. Intrahepatic circulation in liver disease. Semin Liver Dis. 1986 Nov;6(4):277–286. doi: 10.1055/s-2008-1040610. [DOI] [PubMed] [Google Scholar]
- Jaffe V., Alexander B., Mathie R. T. Intrahepatic portal occlusion by microspheres: a new model of portal hypertension in the rat. Gut. 1994 Jun;35(6):815–818. doi: 10.1136/gut.35.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautt W. W., Legare D. J. Passive autoregulation of portal venous pressure: distensible hepatic resistance. Am J Physiol. 1992 Nov;263(5 Pt 1):G702–G708. doi: 10.1152/ajpgi.1992.263.5.G702. [DOI] [PubMed] [Google Scholar]
- Lautt W. W. Mechanism and role of intrinsic regulation of hepatic arterial blood flow: hepatic arterial buffer response. Am J Physiol. 1985 Nov;249(5 Pt 1):G549–G556. doi: 10.1152/ajpgi.1985.249.5.G549. [DOI] [PubMed] [Google Scholar]
- Lee S. S., Girod C., Braillon A., Hadengue A., Lebrec D. Hemodynamic characterization of chronic bile duct-ligated rats: effect of pentobarbital sodium. Am J Physiol. 1986 Aug;251(2 Pt 1):G176–G180. doi: 10.1152/ajpgi.1986.251.2.G176. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Yu P. C., Lee S. D., Tsai Y. T., Kuo J. S., Yang M. C. Effects of reserpine administration in two models of portal hypertension in rats. J Hepatol. 1993 Nov;19(3):413–417. doi: 10.1016/s0168-8278(05)80551-3. [DOI] [PubMed] [Google Scholar]
- Momiyama T., Sim J. A., Brown D. A. Dopamine D1-like receptor-mediated presynaptic inhibition of excitatory transmission onto rat magnocellular basal forebrain neurones. J Physiol. 1996 Aug 15;495(Pt 1):97–106. doi: 10.1113/jphysiol.1996.sp021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myking A. O., Halvorsen J. F. A method for graded portal vein stenosis in rats: survival related to degree of stenosis. Eur Surg Res. 1973;5(6):454–457. doi: 10.1159/000127689. [DOI] [PubMed] [Google Scholar]
- Okuda K., Suzuki K., Musha H., Arimuzu N. Percutaneous transhepatic catheterization of the portal vein for the study of portal hemodynamics and shunts. A preliminary report. Gastroenterology. 1977 Aug;73(2):279–284. [PubMed] [Google Scholar]
- Pérez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983 Jan-Feb;3(1):112–120. doi: 10.1002/hep.1840030118. [DOI] [PubMed] [Google Scholar]
- Sieber C. C., Lopez-Talavera J. C., Groszmann R. J. Role of nitric oxide in the in vitro splanchnic vascular hyporeactivity in ascitic cirrhotic rats. Gastroenterology. 1993 Jun;104(6):1750–1754. doi: 10.1016/0016-5085(93)90655-v. [DOI] [PubMed] [Google Scholar]
- Vorobioff J., Bredfeldt J. E., Groszmann R. J. Increased blood flow through the portal system in cirrhotic rats. Gastroenterology. 1984 Nov;87(5):1120–1126. [PubMed] [Google Scholar]
- Wood A. J., Villeneuve J. P., Branch R. A., Rogers L. W., Shand D. G. Intact hepatocyte theory of impaired drug metabolism in experimental cirrhosis in the rat. Gastroenterology. 1979 Jun;76(6):1358–1362. [PubMed] [Google Scholar]