Abstract
Background—In the lipopolysaccharide (LPS) stimulated peripheral blood monocyte, the precursor form of interleukin 1β (IL-1β, 31 kD) is processed by IL-1β converting enzyme (ICE) to the mature, bioactive form (17 kD). IL-1β is a proinflammatory cytokine which is likely to have a role in the pathogenesis of inflammatory bowel disease (IBD). Aims—To investigate the expression and processing of IL-1β and ICE by tissue macrophages from normal and IBD colonic mucosa. Methods—Mucosal biopsy specimens and lamina propria cells from normal and IBD colons were studied by reverse transcription polymerase chain reaction (RT-PCR), western blot analysis, and ELISA (enzyme linked immunosorbent assay). Results—Normal colonic macrophages synthesised only the precursor form of IL-1β whereas in IBD the mature form was also produced. Similarly, cells from normal colonic mucosa synthesised ICE as the precursor (p45) only, whereas macrophages from IBD colons produced active (p20) ICE. Ac-Tyr-Val-Ala-Asp-CHO, a specific peptide aldehyde inhibitor of ICE, significantly reduced the amount of mature IL-1β released by isolated IBD macrophages (from a median of 1.2 (range 0.78-4.42) ng/ml to 0.43 (0.21-1.6) ng/ml; p<0.01). Conclusions—Exposure of normal colonic macrophages to LPS only induces the production of the precursor form of IL-1β, because the cells fail to activate ICE. In contrast, IBD colonic macrophages are able to activate ICE and hence release mature IL-1β in a manner similar to circulating monocytes. This is consistent with IBD macrophages being recently recruited from the circulating monocyte population. Targeted inhibition of ICE may represent a novel form of therapy in IBD.
Keywords: interleukin 1β; interleukin 1β converting enzyme; macrophages; lipopolysaccharide; ulcerative colitis; Crohn's disease
Full Text
The Full Text of this article is available as a PDF (170.4 KB).
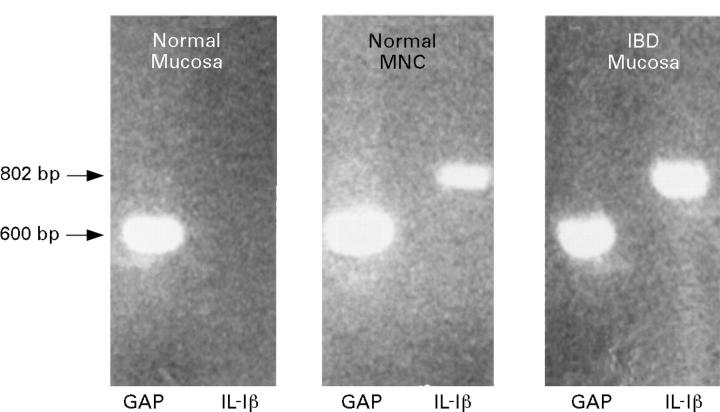
Figure 1 .
Expression of mRNA transcripts for IL-1β and glyceraldehyde-6-phosphate dehydrogenase (GAP) in a normal colonic biopsy specimen; lamina propria mononuclear cells (MNC) isolated from samples of normal colonic mucosa; and a biopsy specimen with active IBD. The figure shows an example of 10/13 normal colonic biopsy samples which did not express transcripts for IL-1β.
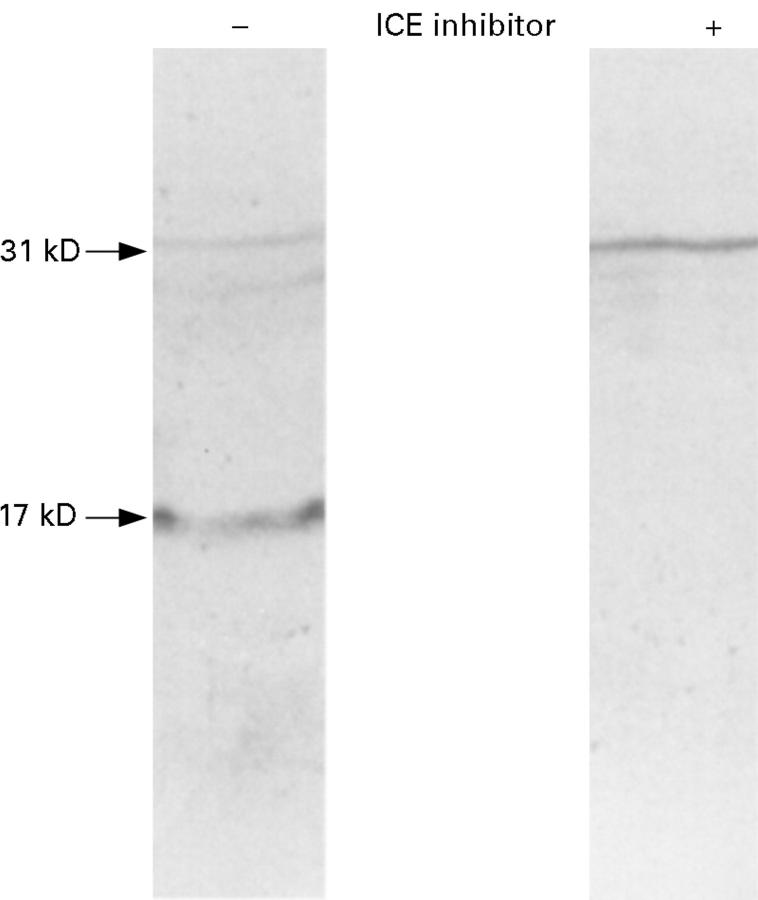
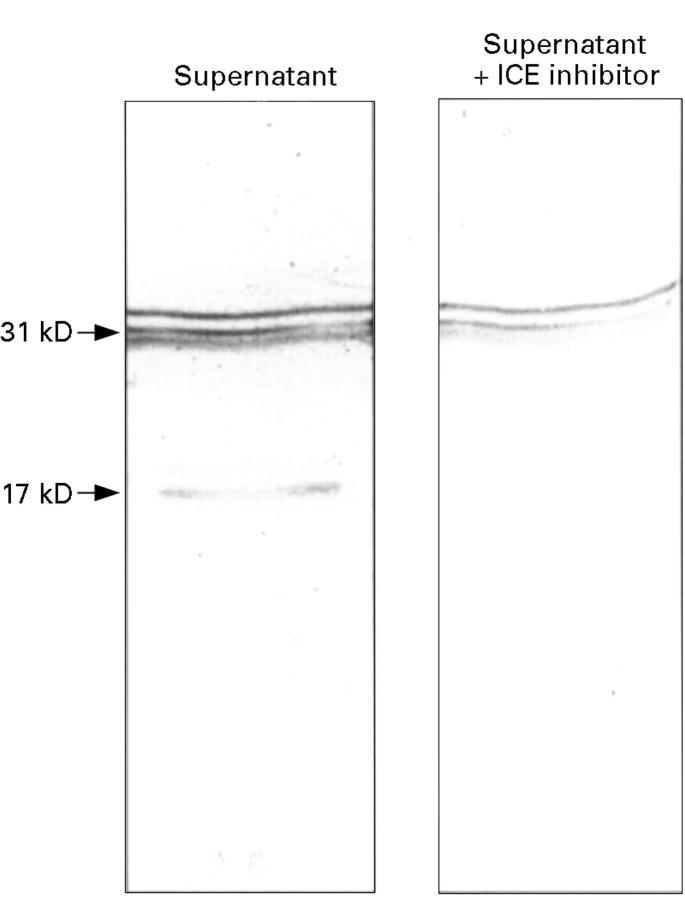
Figure 2 .
Expression of IL-1β protein in LPS stimulated peripheral blood mononuclear cell culture supernatants in the absence (-) and presence (+) of the ICE inhibitor, Ac-Tyr-Val-Ala-Asp-CHO.
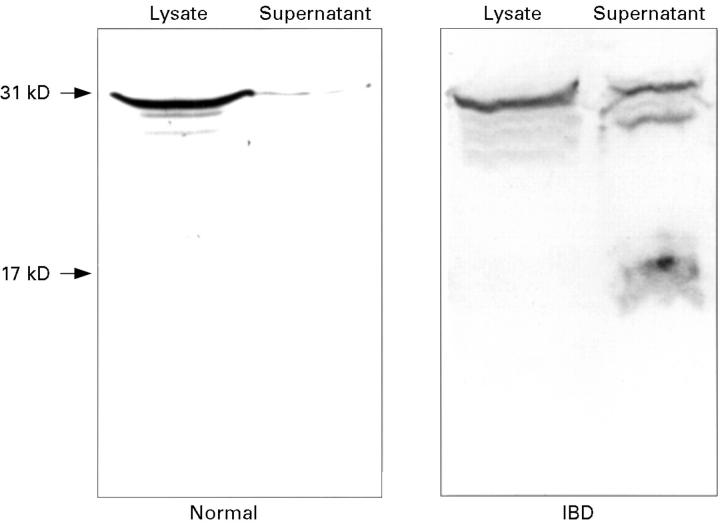
Figure 3 .
Expression of IL-1β protein by LPS stimulated lamina propria cells isolated from normal and IBD colonic mucosa. Cell lysates and supernatants were studied by western blot analysis. In cells obtained from IBD colon, both 31 kD and 17 kD forms of the cytokine were detected in the supernatants. The figure shows representative experiments performed on lamina propria cells obtained from four normal and five IBD colons.
Figure 4 .
Expression of ICE and GAPDH mRNA in biopsy specimens from normal and IBD mucosa.
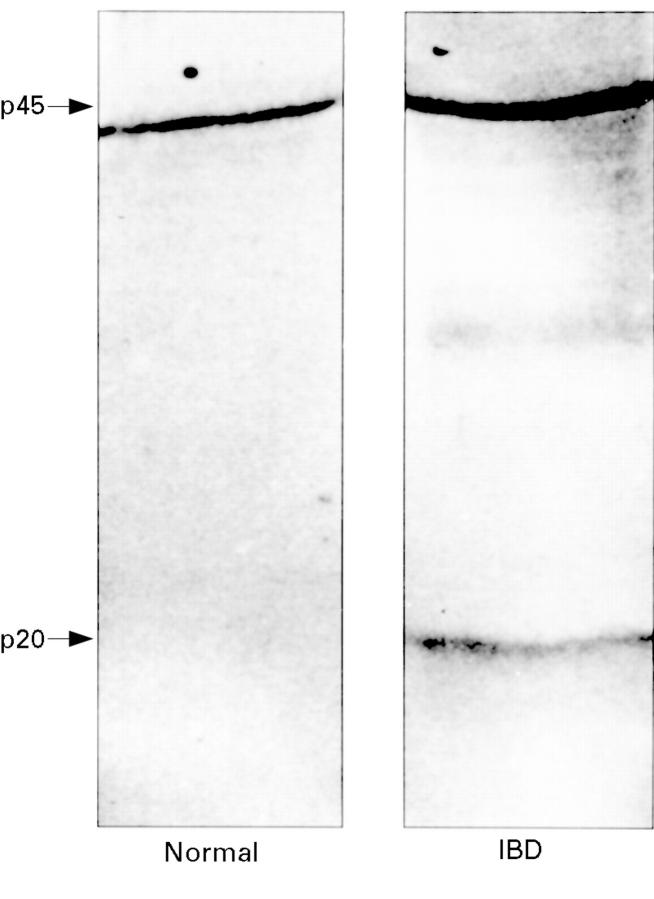
Figure 5 .
Expression of ICE protein by LPS stimulated lamina propria cells isolated from normal and IBD colonic mucosa. In contrast to IBD, the p20 form of ICE was not present in the lysate of cells from normal colonic mucosa. The lighter band between p45 and p20 is likely to represent a 36 kD intermediate cleavage product of p45 (and which is further processed down to p20).38
Figure 6 .
Release of IL-1β protein by IBD lamina propria cells cultured in the presence and absence of ICE inhibitor.
Figure 7 .
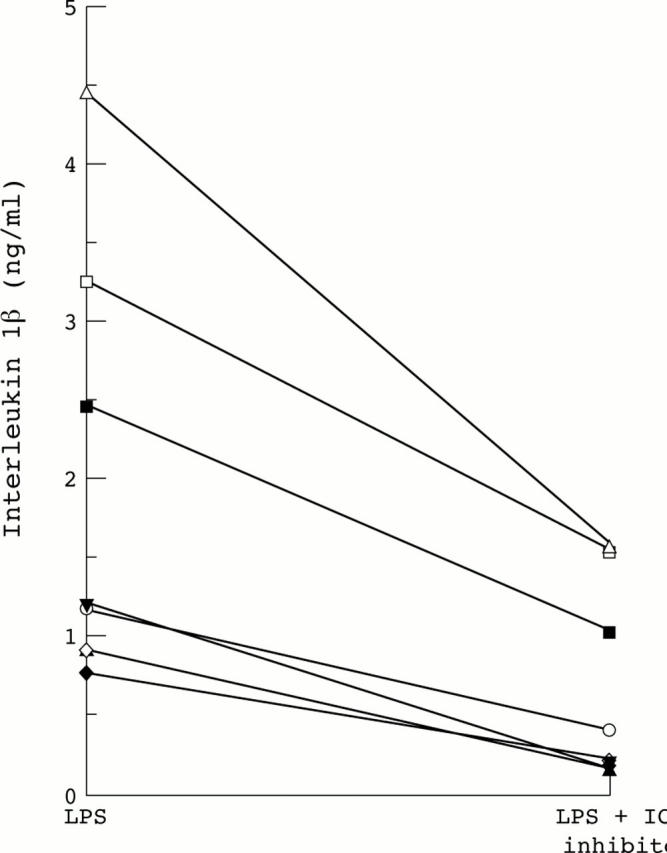
ICE inhibitor (Ac-Tyr-Val-Ala-Asp-CHO) reduces release of mature IL-1β by lamina propria cells isolated from mucosa with active IBD. Paired samples (n=7) of isolated lamina propria cells were cultured with LPS, in the presence or absence of Ac-Tyr-Val-Ala-Asp-CHO.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cappello M., Keshav S., Prince C., Jewell D. P., Gordon S. Detection of mRNAs for macrophage products in inflammatory bowel disease by in situ hybridisation. Gut. 1992 Sep;33(9):1214–1219. doi: 10.1136/gut.33.9.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretti D. P., Kozlosky C. J., Mosley B., Nelson N., Van Ness K., Greenstreet T. A., March C. J., Kronheim S. R., Druck T., Cannizzaro L. A. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992 Apr 3;256(5053):97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- Cominelli F., Nast C. C., Clark B. D., Schindler R., Lierena R., Eysselein V. E., Thompson R. C., Dinarello C. A. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Invest. 1990 Sep;86(3):972–980. doi: 10.1172/JCI114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Gardiner K. R., Halliday M. I., Barclay G. R., Milne L., Brown D., Stephens S., Maxwell R. J., Rowlands B. J. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995 Jun;36(6):897–901. doi: 10.1136/gut.36.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm M. C., Pullman W. E., Bennett G. M., Sullivan P. J., Pavli P., Doe W. F. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J Gastroenterol Hepatol. 1995 Jul-Aug;10(4):387–395. doi: 10.1111/j.1440-1746.1995.tb01589.x. [DOI] [PubMed] [Google Scholar]
- Hogquist K. A., Unanue E. R., Chaplin D. D. Release of IL-1 from mononuclear phagocytes. J Immunol. 1991 Oct 1;147(7):2181–2186. [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- Jung H. C., Eckmann L., Yang S. K., Panja A., Fierer J., Morzycka-Wroblewska E., Kagnoff M. F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995 Jan;95(1):55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruis W., Schussler P., Weinzierl M., Galanos C., Eisenburg J. Circulating lipid A antibodies despite absence of systemic endotoxemia in patients with Crohn's disease. Dig Dis Sci. 1984 Jun;29(6):502–507. doi: 10.1007/BF01296269. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995 Feb 10;80(3):401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Ligumsky M., Simon P. L., Karmeli F., Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990 Jun;31(6):686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Galvin A. M., Gray T., Makh S., McAlindon M. E., Sewell H. F., Podolsky D. K. Migration of human intestinal lamina propria lymphocytes, macrophages and eosinophils following the loss of surface epithelial cells. Clin Exp Immunol. 1997 Aug;109(2):377–386. doi: 10.1046/j.1365-2249.1997.4481346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Lamming C. E., Gallagher A., Hawthorne A. B., Hawkey C. J. 5-Aminosalicylic acid is a potent inhibitor of interleukin 1 beta production in organ culture of colonic biopsy specimens from patients with inflammatory bowel disease. Gut. 1991 Jan;32(1):50–54. doi: 10.1136/gut.32.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Makh S., Hyde S., Gray T., Borriello S. P. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut. 1996 Mar;38(3):337–347. doi: 10.1136/gut.38.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Patel S., Gionchetti P., Vaux D., Jewell D. P. Macrophage subpopulations in lamina propria of normal and inflamed colon and terminal ileum. Gut. 1989 Jun;30(6):826–834. doi: 10.1136/gut.30.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Patel S., Wu K., Jewell D. P. Interleukin 2 receptor expression by macrophages in inflammatory bowel disease. Clin Exp Immunol. 1988 Dec;74(3):382–386. [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K. C., Jewell D. P. Respiratory burst activity of intestinal macrophages in normal and inflammatory bowel disease. Gut. 1989 Oct;30(10):1362–1370. doi: 10.1136/gut.30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K., Jewell D. P. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989 Jun;30(6):835–838. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazlam M. Z., Hodgson H. J. Peripheral blood monocyte cytokine production and acute phase response in inflammatory bowel disease. Gut. 1992 Jun;33(6):773–778. doi: 10.1136/gut.33.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. E., Krasney P. A., Gauvin D. M., Holbrook K. B., Koonz D. J., Abruzzese R. V., Miller R. E., Pagani K. A., Dolle R. E., Ator M. A. Inhibition of mature IL-1 beta production in murine macrophages and a murine model of inflammation by WIN 67694, an inhibitor of IL-1 beta converting enzyme. J Immunol. 1995 Feb 1;154(3):1331–1338. [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Nett-Fiordalisi M., Tomaselli K., Russell J. H., Chaplin D. D. Macrophage apoptosis in the absence of active interleukin-1 beta-converting enzyme. J Leukoc Biol. 1995 Dec;58(6):717–724. doi: 10.1002/jlb.58.6.717. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., Gareau Y., Griffin P. R., Labelle M., Lazebnik Y. A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995 Jul 6;376(6535):37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Perregaux D., Gabel C. A. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994 May 27;269(21):15195–15203. [PubMed] [Google Scholar]
- Ramage P., Cheneval D., Chvei M., Graff P., Hemmig R., Heng R., Kocher H. P., Mackenzie A., Memmert K., Revesz L. Expression, refolding, and autocatalytic proteolytic processing of the interleukin-1 beta-converting enzyme precursor. J Biol Chem. 1995 Apr 21;270(16):9378–9383. doi: 10.1074/jbc.270.16.9378. [DOI] [PubMed] [Google Scholar]
- Reinecker H. C., Loh E. Y., Ringler D. J., Mehta A., Rombeau J. L., MacDermott R. P. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology. 1995 Jan;108(1):40–50. doi: 10.1016/0016-5085(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Riley S. A., Mani V., Goodman M. J., Herd M. E., Dutt S., Turnberg L. A. Comparison of delayed release 5 aminosalicylic acid (mesalazine) and sulphasalazine in the treatment of mild to moderate ulcerative colitis relapse. Gut. 1988 May;29(5):669–674. doi: 10.1136/gut.29.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugtveit J., Haraldsen G., Høgåsen A. K., Bakka A., Brandtzaeg P., Scott H. Respiratory burst of intestinal macrophages in inflammatory bowel disease is mainly caused by CD14+L1+ monocyte derived cells. Gut. 1995 Sep;37(3):367–373. doi: 10.1136/gut.37.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Poulter L. W., Hobbs S., Jewell D. P., Janossy G. Heterogeneity of HLA-DR-positive histiocytes in human intestinal lamina propria: a combined histochemical and immunohistological analysis. J Clin Pathol. 1983 Apr;36(4):379–384. doi: 10.1136/jcp.36.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992 Apr 30;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Wellmann W., Fink P. C., Benner F., Schmidt F. W. Endotoxaemia in active Crohn's disease. Treatment with whole gut irrigation and 5-aminosalicylic acid. Gut. 1986 Jul;27(7):814–820. doi: 10.1136/gut.27.7.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. P., Black J. A., Thomson J. A., Kim E. E., Griffith J. P., Navia M. A., Murcko M. A., Chambers S. P., Aldape R. A., Raybuck S. A. Structure and mechanism of interleukin-1 beta converting enzyme. Nature. 1994 Jul 28;370(6487):270–275. doi: 10.1038/370270a0. [DOI] [PubMed] [Google Scholar]
- Youngman K. R., Simon P. L., West G. A., Cominelli F., Rachmilewitz D., Klein J. S., Fiocchi C. Localization of intestinal interleukin 1 activity and protein and gene expression to lamina propria cells. Gastroenterology. 1993 Mar;104(3):749–758. doi: 10.1016/0016-5085(93)91010-f. [DOI] [PubMed] [Google Scholar]
- di Giovine F. S., Duff G. W. Interleukin 1: the first interleukin. Immunol Today. 1990 Jan;11(1):13–20. doi: 10.1016/0167-5699(90)90005-t. [DOI] [PubMed] [Google Scholar]