Abstract
Background—Oral indomethacin causes villous shortening, microvascular damage, and distortion, which might induce mucosal ischaemia and necrosis. Aims—In order to determine the early events in indomethacin induced jejunal injury we examined the temporal relations between morphological damage and changes in villous blood flow following indomethacin. Methods—In anaesthetised rats, mid jejunal villi were exteriorised in a chamber and observed by fluorescence microscopy. Blood flow in surface capillaries was calculated from velocities and diameters. Indomethacin was applied by both luminal and intravenous routes for 90 minutes, after which the animal was perfusion fixed and the villi were processed for histological examination. Control animals received intravenous or luminal bicarbonate (1.25%). Results—Blood flow slowed in individual villi at 20 minutes, and progressed to complete stasis (in another group) by 45 minutes. Histological examination at 20 minutes revealed microvascular distortion, but no villous shortening: crypt depth:villous height ratios were 0.356 (0.02) in test and 0.386 (0.01) in surrounding villi (p>0.5). At stasis, the villi under study showed epithelial clumping and were shortened: crypt depth:villous height ratios were 0.92 (0.2) in test and 0.42 (0.06) in surrounding villi (p<0.02). Vehicle alone had no effect on either blood flow or histology. Conclusions—Focal slowing of villous blood flow and microvascular distortion precede villus shortening and epithelial disruption, and indicate that damage to surface microvasculature is an early event in indomethacin induced mucosal injury in this model.
Keywords: indomethacin; jejunum; villi; microcirculation; endothelium; microthrombi
Full Text
The Full Text of this article is available as a PDF (201.6 KB).
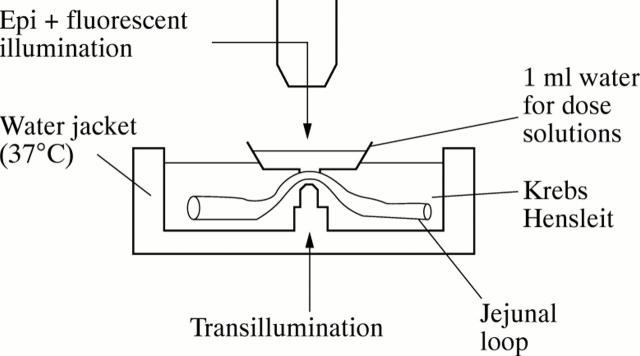
Figure 1 .
Apparatus used for in vivo microscopy. The water jacket is attached to the side of the animal and a loop of jejunum exteriorised and placed on an observation peg. A small water bath is then placed using micromanipulators onto the mucosal surface of the jejunum and test solutions placed into this bath.
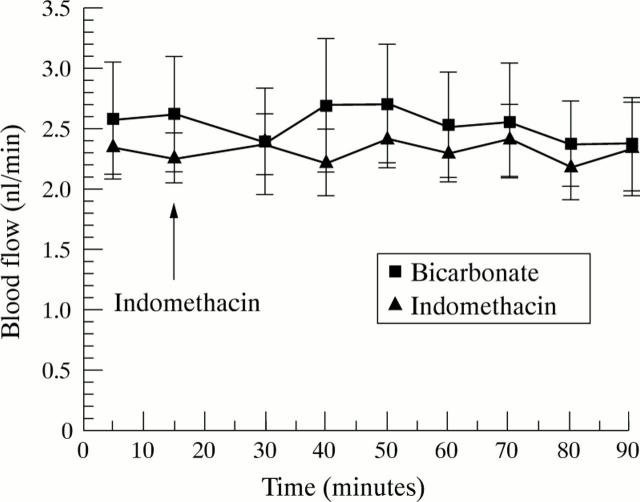
Figure 2 .
Effect of either luminal or intravenous indomethacin (100 µg/ml, 2.8 × 10−4M) and bicarbonate control (0.034%) on jejunal villous blood flow. Each point expressed as mean (SEM) of five rats (p=0.9, NS).
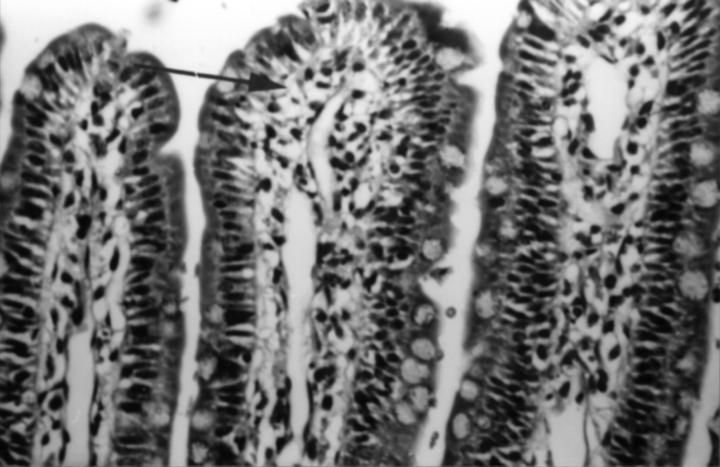
Figure 3 .
Normal jejunal villous tip from a rat which received luminal indomethacin (100 µg/ml, 2.8 × 10−4M) and was perfusion fixed 90 minutes after dosing. Subepithelial capillaries are cleared of blood (arrow) indicating no vascular occlusion/blockage, with good preservation of villous architecture. Haematoxylin and eosin, original magnification ×250.
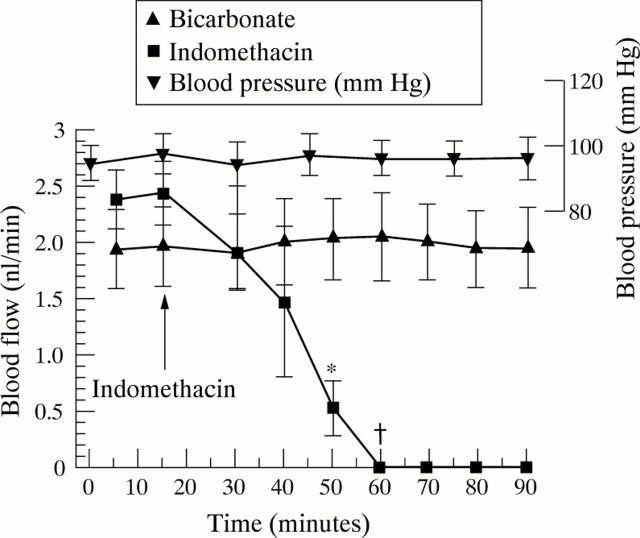
Figure 4 .
Effect of a combined intravenous and luminal dose of indomethacin (100 µg/ml, 2.8 × 10−4M) and bicarbonate control on jejunal villous blood flow. Each point expressed as mean (SEM) of five rats. *p<0.02, †p<0.005.
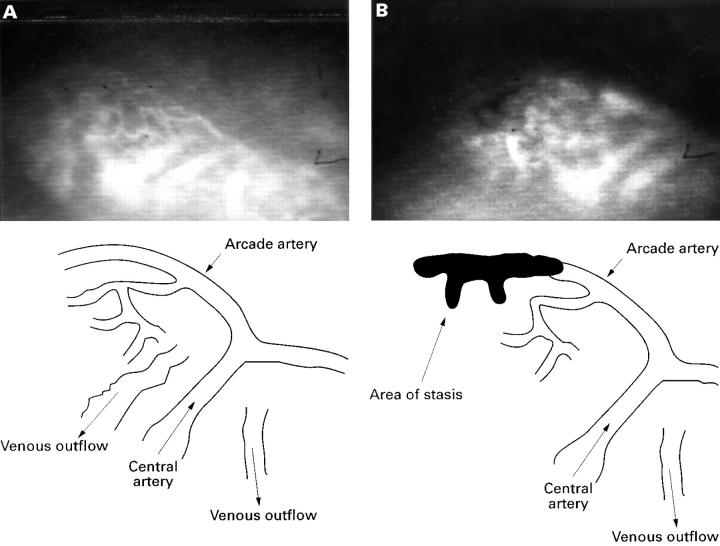
Figure 5 .
(A) Video image obtained from a normal in vivo villus prior to application of indomethacin; the vasculature is highlighted with FITC-dextran in the plasma. Bottom left: line diagram showing the vascular anatomy of the villus in A. Notice the central artery ascending the centre of the villus and dividing at the tip as a T junction to form the arcade artery. This runs along to the left along the tip to supply the left half of the villus, and along the right tip for the right half of the villus. (B) The same villus after application of indomethacin: the darkened area in the upper left half is an area of blood stasis; this is shown more clearly in the line drawing.
Figure 6 .
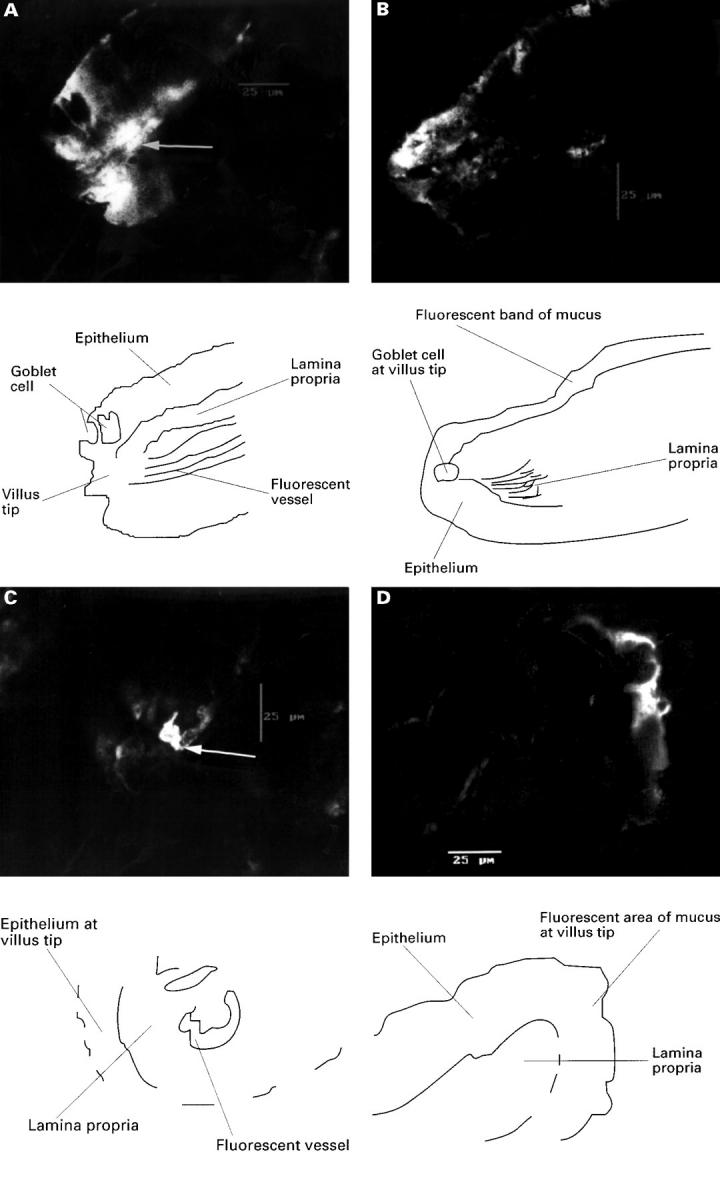
(A) An isolated villus from rat jejunum after combined indomethacin, taken from the left half of the villus which showed stasis and vessel hyperfluorescence (arrow), at which point the villus was perfuse fixed; the area of fluoresence to the left of this vessel is epithelium; original magnification ×600. This is more clearly seen in the line drawing. (B) Image from other right side of the villus which had normal flow and no fluorescence except for surface mucus; original magnification ×600. This is more clearly shown in the line drawing. (C) Transverse confocal image of an isolated villus from rat jejunum after combined luminal (100 µg/ml, 2.8 × 10−4M) and intravenous (15 mg/kg) indomethacin, and perfusion fixed as blood flow slowed at 30 minutes. Image is taken from the right side of the villus which exhibited slowing of flow and hyperfluorescence of the arcade artery (arrow), which is surrounded by epithelium showing slight fluoresence due to surface mucus; original magnification ×600. This is more clearly shown in the line drawing. (D) The left side of this villus which maintained normal flow. The only fluorescence again comes from surface mucus on the epithelium; original magnification ×600. This is more clearly shown in the line drawing.
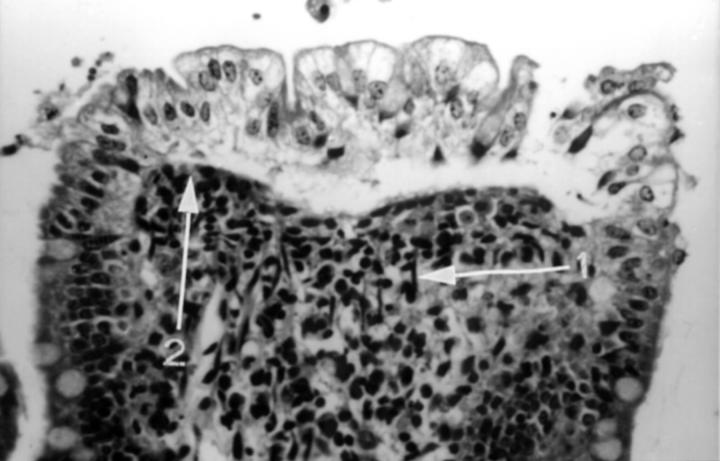
Figure 7 .
Villous tip from rat jejunum showing blood stasis after combined luminal (100 µg/ml, 2.8 × 10−4M) and intravenous (15 mg/kg) indomethacin. The surface epithelium is beginning to lift and fall away from the lamina propria. There is contraction of surface smooth muscle cells manifest by prominent contractile elements (arrow 1) causing occlusion of surface vessels (arrow 2); there is also degeneration of the lamina propria; original magnification ×250.
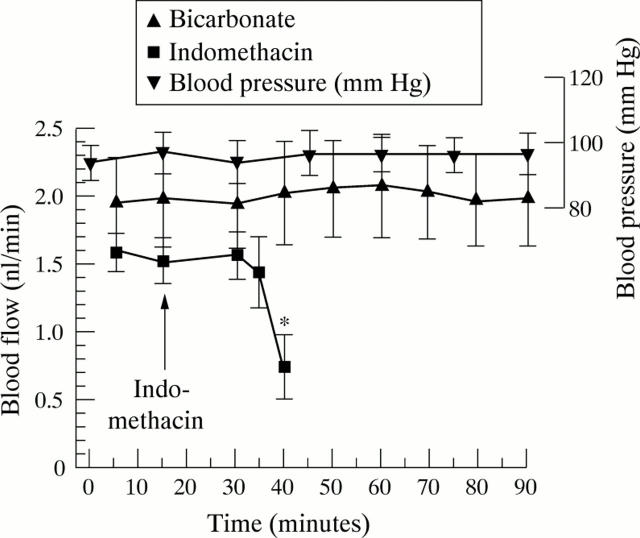
Figure 8 .
Effect of a combined intravenous and luminal dose of indomethacin (100 µg/ml) and bicarbonate control on jejunal villous blood flow. Each point expressed as mean (SEM) of five rats. *p<0.03.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aabakken L., Osnes M. Non-steroidal anti-inflammatory drug-induced disease in the distal ileum and large bowel. Scand J Gastroenterol Suppl. 1989;163:48–55. doi: 10.3109/00365528909091175. [DOI] [PubMed] [Google Scholar]
- Allison M. C., Howatson A. G., Torrance C. J., Lee F. D., Russell R. I. Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N Engl J Med. 1992 Sep 10;327(11):749–754. doi: 10.1056/NEJM199209103271101. [DOI] [PubMed] [Google Scholar]
- Anthony A., Dhillon A. P., Nygard G., Hudson M., Piasecki C., Strong P., Trevethick M. A., Clayton N. M., Jordan C. C., Pounder R. E. Early histological features of small intestinal injury induced by indomethacin. Aliment Pharmacol Ther. 1993 Feb;7(1):29–39. doi: 10.1111/j.1365-2036.1993.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Anthony A., Pounder R. E., Dhillon A. P., Wakefield A. J. Vascular anatomy defines sites of indomethacin induced jejunal ulceration along the mesenteric margin. Gut. 1997 Dec;41(6):763–770. doi: 10.1136/gut.41.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Smethurst P., Fenn C. G., Lee C. E., Menzies I. S., Levi A. J. Misoprostol reduces indomethacin-induced changes in human small intestinal permeability. Dig Dis Sci. 1989 Mar;34(3):407–411. doi: 10.1007/BF01536263. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Prouse P., Smethurst P., Smith T., Levi S., Gumpel M. J., Levi A. J. Blood and protein loss via small-intestinal inflammation induced by non-steroidal anti-inflammatory drugs. Lancet. 1987 Sep 26;2(8561):711–714. doi: 10.1016/s0140-6736(87)91075-0. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Smith T., Prouse P., Williams P., Smethurst P., Delacey G., Gumpel M. J., Levi A. J. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology. 1987 Sep;93(3):480–489. doi: 10.1016/0016-5085(87)90909-7. [DOI] [PubMed] [Google Scholar]
- Crotty B., Hoang P., Dalton H. R., Jewell D. P. Salicylates used in inflammatory bowel disease and colchicine impair interferon-gamma induced HLA-DR expression. Gut. 1992 Jan;33(1):59–64. doi: 10.1136/gut.33.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigen L. P., King L. W., Ray J., Beckett W., Kadowitz P. J. Differential effects of ibuprofen and indomethacin in the regional circulation of the dog. J Pharmacol Exp Ther. 1981 Dec;219(3):679–684. [PubMed] [Google Scholar]
- Gaffney G. R., Williamson H. E. Effect of indomethacin and meclofenamate on canine mesenteric and celiac blood flow. Res Commun Chem Pathol Pharmacol. 1979 Jul;25(1):165–168. [PubMed] [Google Scholar]
- Gerkens J. F., Flexner C., Oates J. A., Shand D. G. Prostaglandin and histamine involvement in the gastric vasodilator action of pentagastrin. J Pharmacol Exp Ther. 1977 May;201(2):421–426. [PubMed] [Google Scholar]
- Gerkens J. F., Shand D. G., Flexner C., Nies A. S., Oates J. A., Data J. L. Effect of indomethacin and aspirin on gastric blood flow and acid secretion. J Pharmacol Exp Ther. 1977 Dec;203(3):646–652. [PubMed] [Google Scholar]
- Holliger C., Radzyner M., Knoblauch M. Effects of glucagon, vasoactive intestinal peptide, and vasopressin on villous microcirculation and superior mesenteric artery blood flow of the rat. Gastroenterology. 1983 Nov;85(5):1036–1043. [PubMed] [Google Scholar]
- Knoblauch M., Holliger C. Mikrozirkulationsstudien am Villus des Rattendünndarmes in vivo. Schweiz Med Wochenschr. 1977 Oct 8;107(40):1391–1399. [PubMed] [Google Scholar]
- Miller F. N., Sims D. E., Schuschke D. A., Abney D. L. Differentiation of light-dye effects in the microcirculation. Microvasc Res. 1992 Sep;44(2):166–184. doi: 10.1016/0026-2862(92)90078-4. [DOI] [PubMed] [Google Scholar]
- Morris A. J., Madhok R., Sturrock R. D., Capell H. A., MacKenzie J. F. Enteroscopic diagnosis of small bowel ulceration in patients receiving non-steroidal anti-inflammatory drugs. Lancet. 1991 Mar 2;337(8740):520–520. doi: 10.1016/0140-6736(91)91300-j. [DOI] [PubMed] [Google Scholar]
- Morris A. J., Wasson L. A., MacKenzie J. F. Small bowel enteroscopy in undiagnosed gastrointestinal blood loss. Gut. 1992 Jul;33(7):887–889. doi: 10.1136/gut.33.7.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff M., Métry J. M., Frick P., Anliker M., Knoblauch M. The microvasculature of the small-intestinal mucosa of the rat: quantification of hemodynamic effects of topically applied cimetidine, ranitidine, somatostatin, and vasopressin. Scand J Gastroenterol Suppl. 1985;112:6–11. doi: 10.3109/00365528509092207. [DOI] [PubMed] [Google Scholar]
- Nygård G., Anthony A., Piasecki C., Trevethick M. A., Hudson M., Dhillon A. P., Pounder R. E., Wakefield A. J. Acute indomethacin-induced jejunal injury in the rat: early morphological and biochemical changes. Gastroenterology. 1994 Mar;106(3):567–575. doi: 10.1016/0016-5085(94)90687-4. [DOI] [PubMed] [Google Scholar]
- Pries A. R. A versatile video image analysis system for microcirculatory research. Int J Microcirc Clin Exp. 1988 Nov;7(4):327–345. [PubMed] [Google Scholar]
- Sarelius I. H., Duling B. R. Direct measurement of microvessel hematocrit, red cell flux, velocity, and transit time. Am J Physiol. 1982 Dec;243(6):H1018–H1026. doi: 10.1152/ajpheart.1982.243.6.H1018. [DOI] [PubMed] [Google Scholar]
- Sarelius I. H. Microcirculation in striated muscle after acute reduction in systemic hematocrit. Respir Physiol. 1989 Oct;78(1):7–17. doi: 10.1016/0034-5687(89)90138-2. [DOI] [PubMed] [Google Scholar]
- Seki J. Fiber-optic laser-Doppler anemometer microscope developed for the measurement of microvascular red cell velocity. Microvasc Res. 1990 Nov;40(3):302–316. doi: 10.1016/0026-2862(90)90029-q. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Igarashi J., Yamashiro Y., Yabuta K. Effects of indomethacin on jejunal mucosal blood flow in the infant rat. Eur J Pediatr. 1995 Jul;154(7):592–593. doi: 10.1007/BF02074849. [DOI] [PubMed] [Google Scholar]
- Slater C., House S. D. Effects of nonsteroidal anti-inflammatory drugs on microvascular dynamics. Microvasc Res. 1993 Mar;45(2):166–179. doi: 10.1006/mvre.1993.1016. [DOI] [PubMed] [Google Scholar]
- Svanes K., Ulven A. Gastric ulceration associated with experimental vascular occlusion. Digestion. 1977;15(6):517–525. doi: 10.1159/000198042. [DOI] [PubMed] [Google Scholar]
- Taha A. S., Angerson W., Nakshabendi I., Beekman H., Morran C., Sturrock R. D., Russell R. I. Gastric and duodenal mucosal blood flow in patients receiving non-steroidal anti-inflammatory drugs--influence of age, smoking, ulceration and Helicobacter pylori. Aliment Pharmacol Ther. 1993 Feb;7(1):41–45. doi: 10.1111/j.1365-2036.1993.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Kauffman G. L., Moncada S. Vasoconstriction with thromboxane A2 induces ulceration of the gastric mucosa. Nature. 1981 Jul 30;292(5822):472–474. doi: 10.1038/292472a0. [DOI] [PubMed] [Google Scholar]