Abstract
Background—The mechanisms responsible for the initiation of alcoholic pancreatitis remain elusive. However, there is an increasing body of evidence that reactive oxygen species play a role in both acute and chronic pancreatitis. In the liver, cytochrome P4502E1 (CYP2E1, the inducible ethanol metabolising enzyme) is one of the proposed pathways by which ethanol induces oxidative stress. Aims—To determine whether CYP2E1 is present in the pancreas and, if so, whether it is inducible by chronic ethanol feeding. Methods—Eighteen male Sprague-Dawley rats were pair fed liquid diets with or without ethanol as 36% of energy for four weeks. CYP2E1 levels were determined by western blotting of microsomal protein from both pancreas and liver. Messenger RNA (mRNA) levels for CYP2E1 were quantified using dot blots of total pancreatic RNA. Results—CYP2E1 was found in the pancreas. Furthermore, the amount of CYP2E1 was greater in the pancreas of rats fed ethanol compared with controls (mean increase over controls 5.1-fold, 95% confidence intervals 2.4 to 7.7, p<0.02). In the liver, induction by ethanol of CYP2E1 was similar (mean increase over controls 7.9-fold, 95% confidence intervals 5.2 to 10.6, p<0.005). Pancreatic mRNA levels for CYP2E1 were similar in ethanol fed and control rats. Conclusions—CYP2E1 is present in the rat pancreas and is inducible by chronic ethanol administration. Induction of pancreatic CYP2E1 is not regulated at the mRNA level. The metabolism of ethanol via CYP2E1 may contribute to oxidative stress in the pancreas during chronic ethanol consumption.
Keywords: cytochrome P4502E1; rat pancreas; chronic ethanol administration
Full Text
The Full Text of this article is available as a PDF (141.1 KB).
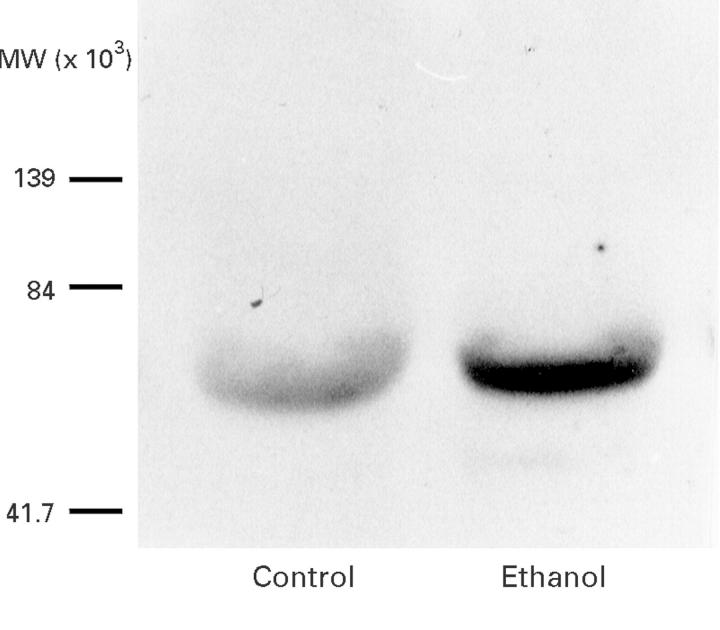
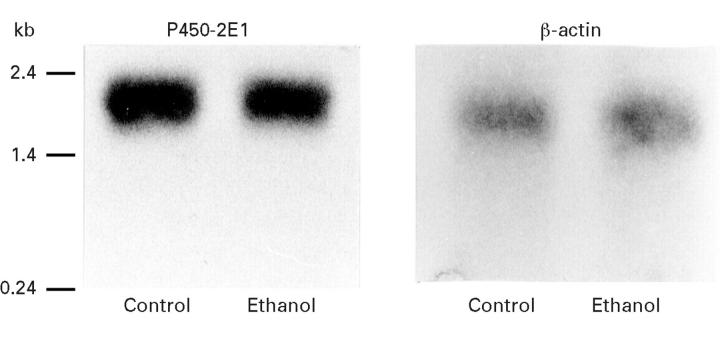
Figure 1 .
Representative western blot of pancreatic microsomal protein showing the presence of cytochrome P4502E1 in control pancreas and induction of this protein due to chronic ethanol administration.
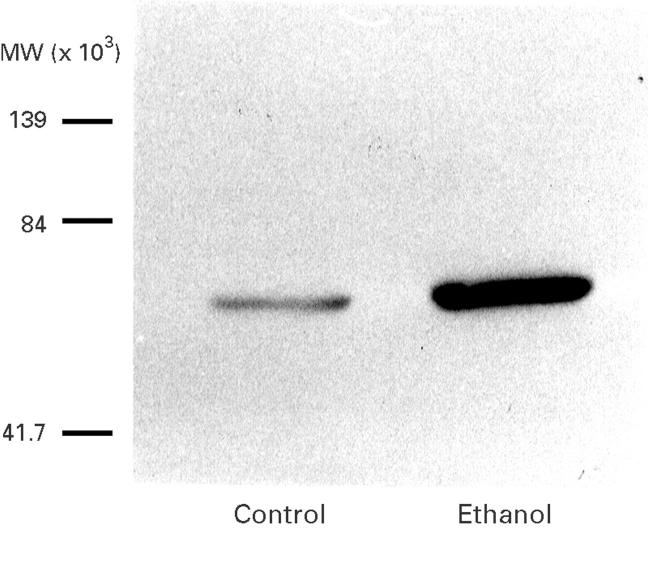
Figure 2 .
Representative western blot of hepatic microsomal protein showing the presence of cytochrome P4502E1 in control liver and induction of this protein due to chronic ethanol administration.
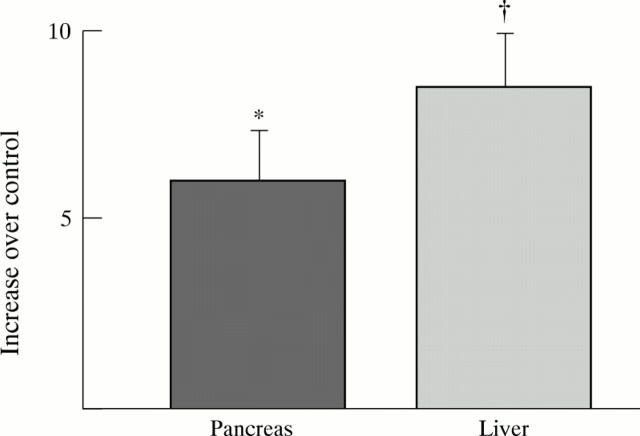
Figure 3 .
Induction of CYP2E1 in pancreas and liver of rats fed ethanol. Results expressed as x fold increase over control, calculated from arbitrary densitometer units per µg of microsomal protein. n=9 pairs. *p<0.02, †p<0.005 compared with control.
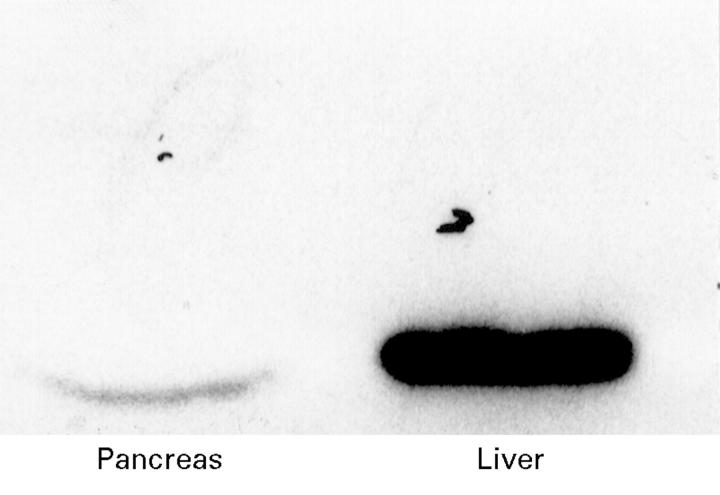
Figure 4 .
Representative western blot comparing the induction of CYP2E1 in the pancreas and liver during chronic ethanol exposure.
Figure 5 .
Representative autoradiographs of northern blots for β actin and P4502E1 mRNA showing specificity of the probes.
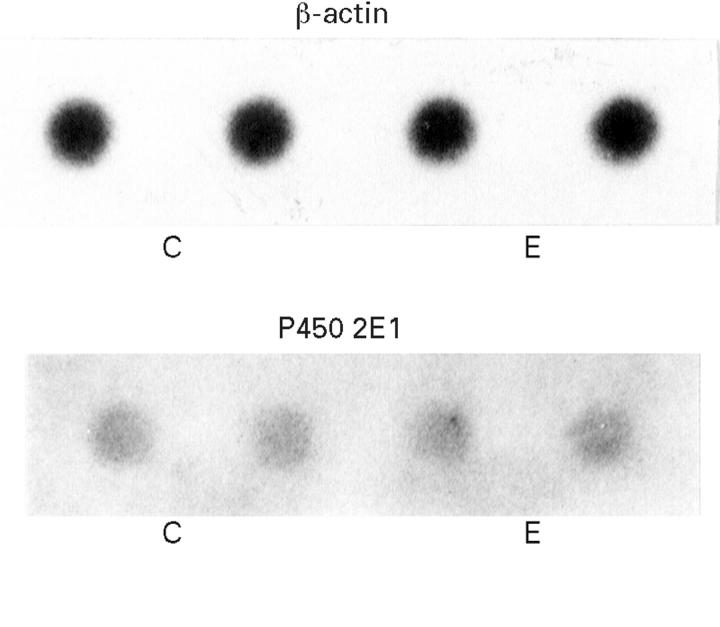
Figure 6 .
Representative autoradiographs of RNA dot blots from control (C) and ethanol fed (E) animals.
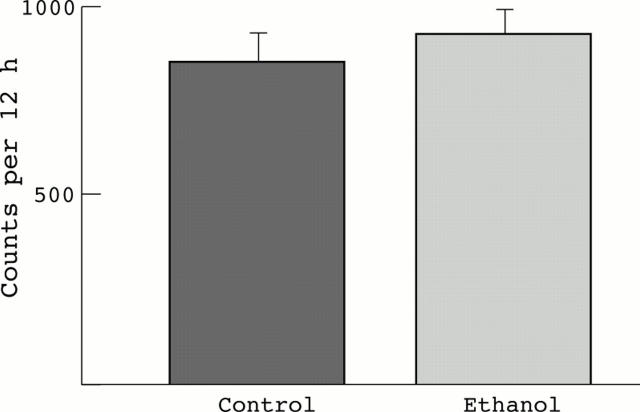
Figure 7 .
Comparison of CYP2E1 mRNA dot blots, determined by microchannel array detection. n=8 pairs (NS).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altomare E., Grattagliano I., Vendemiale G., Palmieri V., Palasciano G. Acute ethanol administration induces oxidative changes in rat pancreatic tissue. Gut. 1996 May;38(5):742–746. doi: 10.1136/gut.38.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte M. V., Wilson J. S., McCaughan G. W., Korsten M. A., Haber P. S., Norton I. D., Pirola R. C. Ethanol-induced alterations in messenger RNA levels correlate with glandular content of pancreatic enzymes. J Lab Clin Med. 1995 May;125(5):634–640. [PubMed] [Google Scholar]
- Braganza J. M., Schofield D., Snehalatha C., Mohan V. Micronutrient antioxidant status in tropical compared with temperate-zone chronic pancreatitis. Scand J Gastroenterol. 1993 Dec;28(12):1098–1104. doi: 10.3109/00365529309098316. [DOI] [PubMed] [Google Scholar]
- Braganza J. M., Scott P., Bilton D., Schofield D., Chaloner C., Shiel N., Hunt L. P., Bottiglieri T. Evidence for early oxidative stress in acute pancreatitis. Clues for correction. Int J Pancreatol. 1995 Feb;17(1):69–81. doi: 10.1007/BF02788361. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ekström G., Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1). Biochem Pharmacol. 1989 Apr 15;38(8):1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- Foster J. R., Idle J. R., Hardwick J. P., Bars R., Scott P., Braganza J. M. Induction of drug-metabolizing enzymes in human pancreatic cancer and chronic pancreatitis. J Pathol. 1993 Apr;169(4):457–463. doi: 10.1002/path.1711690412. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988 Dec;40(4):243–288. [PubMed] [Google Scholar]
- Guyan P. M., Uden S., Braganza J. M. Heightened free radical activity in pancreatitis. Free Radic Biol Med. 1990;8(4):347–354. doi: 10.1016/0891-5849(90)90100-w. [DOI] [PubMed] [Google Scholar]
- Haber P., Wilson J., Apte M., Korsten M., Pirola R. Individual susceptibility to alcoholic pancreatitis: still an enigma. J Lab Clin Med. 1995 Mar;125(3):305–312. [PubMed] [Google Scholar]
- Hayashi S., Watanabe J., Kawajiri K. Genetic polymorphisms in the 5'-flanking region change transcriptional regulation of the human cytochrome P450IIE1 gene. J Biochem. 1991 Oct;110(4):559–565. doi: 10.1093/oxfordjournals.jbchem.a123619. [DOI] [PubMed] [Google Scholar]
- Iimuro Y., Bradford B. U., Gao W., Kadiiska M., Mason R. P., Stefanovic B., Brenner D. A., Thurman R. G. Detection of alpha-hydroxyethyl free radical adducts in the pancreas after chronic exposure to alcohol in the rat. Mol Pharmacol. 1996 Sep;50(3):656–661. [PubMed] [Google Scholar]
- Johansson I., Ekström G., Scholte B., Puzycki D., Jörnvall H., Ingelman-Sundberg M. Ethanol-, fasting-, and acetone-inducible cytochromes P-450 in rat liver: regulation and characteristics of enzymes belonging to the IIB and IIE gene subfamilies. Biochemistry. 1988 Mar 22;27(6):1925–1934. doi: 10.1021/bi00406a019. [DOI] [PubMed] [Google Scholar]
- Kato S., Onda M., Matsukura N., Tokunaga A., Tajiri T., Kim D. Y., Tsuruta H., Matsuda N., Yamashita K., Shields P. G. Cytochrome P4502E1 (CYP2E1) genetic polymorphism in a case-control study of gastric cancer and liver disease. Pharmacogenetics. 1995;5(Spec No):S141–S144. doi: 10.1097/00008571-199512001-00016. [DOI] [PubMed] [Google Scholar]
- Kim S. G., Novak R. F. Induction of rat hepatic P450IIE1 (CYP 2E1) by pyridine: evidence for a role of protein synthesis in the absence of transcriptional activation. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1072–1079. doi: 10.1016/0006-291x(90)90976-t. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M., Sorrell M. F. Experimental methods of ethanol administration. Hepatology. 1989 Oct;10(4):501–510. doi: 10.1002/hep.1840100417. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. The feeding of ethanol in liquid diets. Alcohol Clin Exp Res. 1986 Oct;10(5):550–553. doi: 10.1111/j.1530-0277.1986.tb05140.x. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. The role of the hepatic microsomal ethanol oxidizing system (MEOS) for ethanol metabolism in vivo. J Pharmacol Exp Ther. 1972 May;181(2):279–287. [PubMed] [Google Scholar]
- Meldolesi J., Jamieson J. D., Palade G. E. Composition of cellular membranes in the pancreas of the guinea pig. 3. Enzymatic activities. J Cell Biol. 1971 Apr;49(1):150–158. doi: 10.1083/jcb.49.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuschwander-Tetri B. A., Ferrell L. D., Sukhabote R. J., Grendell J. H. Glutathione monoethyl ester ameliorates caerulein-induced pancreatitis in the mouse. J Clin Invest. 1992 Jan;89(1):109–116. doi: 10.1172/JCI115550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederau C., Niederau M., Borchard F., Ude K., Lüthen R., Strohmeyer G., Ferrell L. D., Grendell J. H. Effects of antioxidants and free radical scavengers in three different models of acute pancreatitis. Pancreas. 1992;7(4):486–496. doi: 10.1097/00006676-199207000-00011. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M., Kitteringham N. R., Quest L. J., Allott R. L., Green V. J., Gilmore I. T., Park B. K. Genetic polymorphism of cytochrome P4502E1 and risk of alcoholic liver disease in Caucasians. Pharmacogenetics. 1995 Dec;5(6):351–357. doi: 10.1097/00008571-199512000-00003. [DOI] [PubMed] [Google Scholar]
- Sanfey H., Bulkley G. B., Cameron J. L. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg. 1984 Oct;200(4):405–413. doi: 10.1097/00000658-198410000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey H., Sarr M. G., Bulkley G. B., Cameron J. L. Oxygen-derived free radicals and acute pancreatitis: a review. Acta Physiol Scand Suppl. 1986;548:109–118. [PubMed] [Google Scholar]
- Schoenberg M. H., Büchler M., Beger H. G. Oxygen radicals in experimental acute pancreatitis. Hepatogastroenterology. 1994 Aug;41(4):313–319. [PubMed] [Google Scholar]
- Schoenberg M. H., Büchler M., Gaspar M., Stinner A., Younes M., Melzner I., Bültmann B., Beger H. G. Oxygen free radicals in acute pancreatitis of the rat. Gut. 1990 Oct;31(10):1138–1143. doi: 10.1136/gut.31.10.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M. H., Büchler M., Pietrzyk C., Uhl W., Birk D., Eisele S., Marzinzig M., Beger H. G. Lipid peroxidation and glutathione metabolism in chronic pancreatitis. Pancreas. 1995 Jan;10(1):36–43. doi: 10.1097/00006676-199501000-00005. [DOI] [PubMed] [Google Scholar]
- Schoenberg M. H., Büchler M., Younes M., Kirchmayr R., Brückner U. B., Beger H. G. Effect of antioxidant treatment in rats with acute hemorrhagic pancreatitis. Dig Dis Sci. 1994 May;39(5):1034–1040. doi: 10.1007/BF02087555. [DOI] [PubMed] [Google Scholar]
- Song B. J., Veech R. L., Park S. S., Gelboin H. V., Gonzalez F. J. Induction of rat hepatic N-nitrosodimethylamine demethylase by acetone is due to protein stabilization. J Biol Chem. 1989 Feb 25;264(6):3568–3572. [PubMed] [Google Scholar]
- Steinberg W., Tenner S. Acute pancreatitis. N Engl J Med. 1994 Apr 28;330(17):1198–1210. doi: 10.1056/NEJM199404283301706. [DOI] [PubMed] [Google Scholar]
- Teare J. P., Greenfield S. M., Watson D., Punchard N. A., Miller N., Rice-Evans C. A., Thompson R. P. Lipid peroxidation in rats chronically fed ethanol. Gut. 1994 Nov;35(11):1644–1647. doi: 10.1136/gut.35.11.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M., Lasker J. M., Shimizu M., Rosman A. S., Lieber C. S. The intralobular distribution of ethanol-inducible P450IIE1 in rat and human liver. Hepatology. 1989 Oct;10(4):437–446. doi: 10.1002/hep.1840100407. [DOI] [PubMed] [Google Scholar]
- Uden S., Acheson D. W., Reeves J., Worthington H. V., Hunt L. P., Brown S., Braganza J. M. Antioxidants, enzyme induction, and chronic pancreatitis: a reappraisal following studies in patients on anticonvulsants. Eur J Clin Nutr. 1988 Jul;42(7):561–569. [PubMed] [Google Scholar]