Abstract
Background—Secretory immunity is a major defence mechanism against infections at mucosal surfaces which are common in HIV infected patients. Aims—To analyse intestinal immunoglobulin production in HIV infection in comparison with that in saliva and serum. Patients and methods—Immunoglobulin G (IgG), A (IgA), and M (IgM) concentrations were determined in supernatants of short term cultured duodenal biopsy samples, serum, and saliva from HIV infected patients (n = 28) and controls (n = 14) by radial immunodiffusion. Results—IgG was increased in the supernatants of short term cultured biopsy samples and saliva from HIV infected patients compared with controls (p<0.01), but IgA and IgM levels were normal. In contrast, both IgG and IgA concentrations in serum were higher in HIV infected patients than in controls (p<0.002). No correlation was found between IgA produced by duodenal biopsy specimens and serum IgA. Conclusion—Abnormalities in mucosal immunoglobulin production in HIV infection were suprisingly small, indicating that specific secretory immunity rather than quantitative immunoglobulin production may be impaired. However, increased production of IgG could contribute to mucosal inflammation by complement activation. Our findings of normal mucosal IgA production and the lack of correlation between serum and mucosal IgA argues against an intestinal origin for the increased serum IgA levels in HIV infected patients.
Keywords: mucosal immunity; HIV infection; intestinal antibodies
Full Text
The Full Text of this article is available as a PDF (130.0 KB).
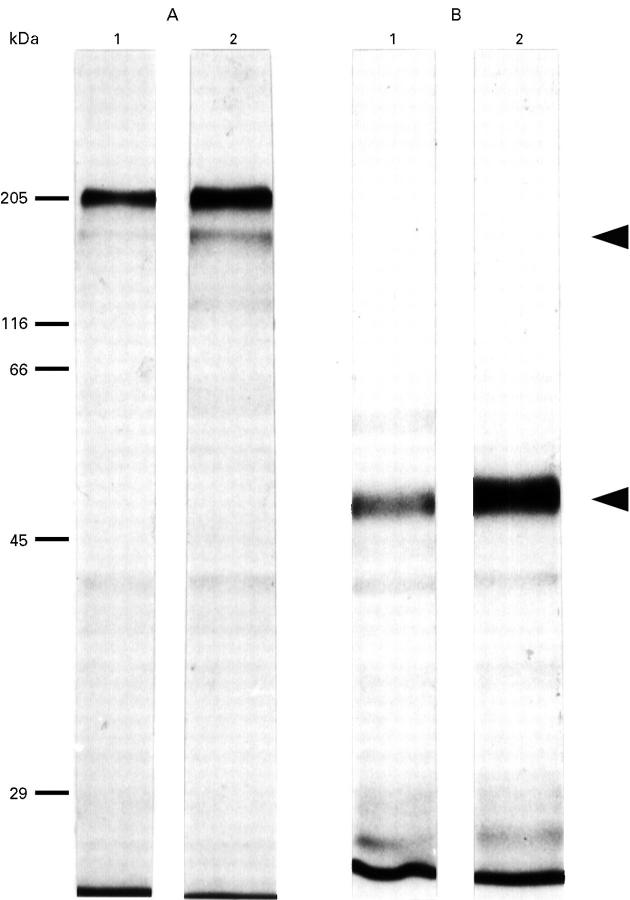
Figure 1 .
Autoradiography of short term cultured biopsy supernatants after 35S-labelling of immunoglobulin disulphide bonds. The two autoradiographs (A, non-reducing conditions; B, reducing conditions) show in lane 1 the Protein A/Sepharose precipitate and in lane 2 the Protein A/Sepharose/anti-IgA precipitate. Under non-reducing conditions the immunoglobulin remains intact and migrates at 160 kDa. Under reducing conditions the immunoglobulin is cleaved into the heavy chain migrating at 55 kDa, seen here, and a band of 25 kDa, not seen on the 10% gel used here which separates molecules in the molecular mass range 29-200 kDa.
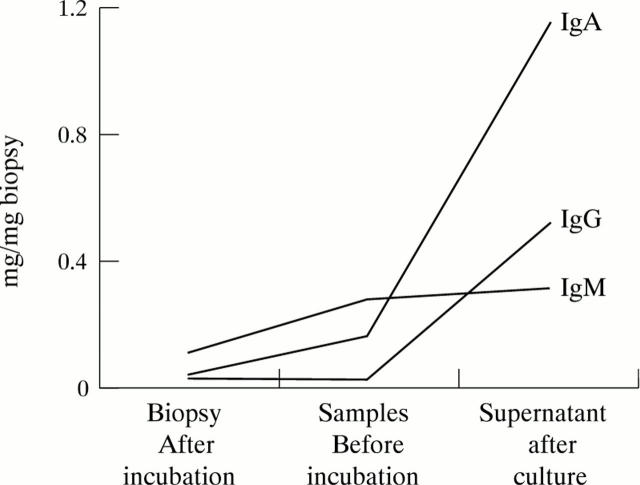
Figure 2 .
Representative example of immunoglobulin isotype (IgG, IgA, IgM) concentrations in 48 hour culture supernatant, and in biopsy samples before and after culture.
Figure 3 .
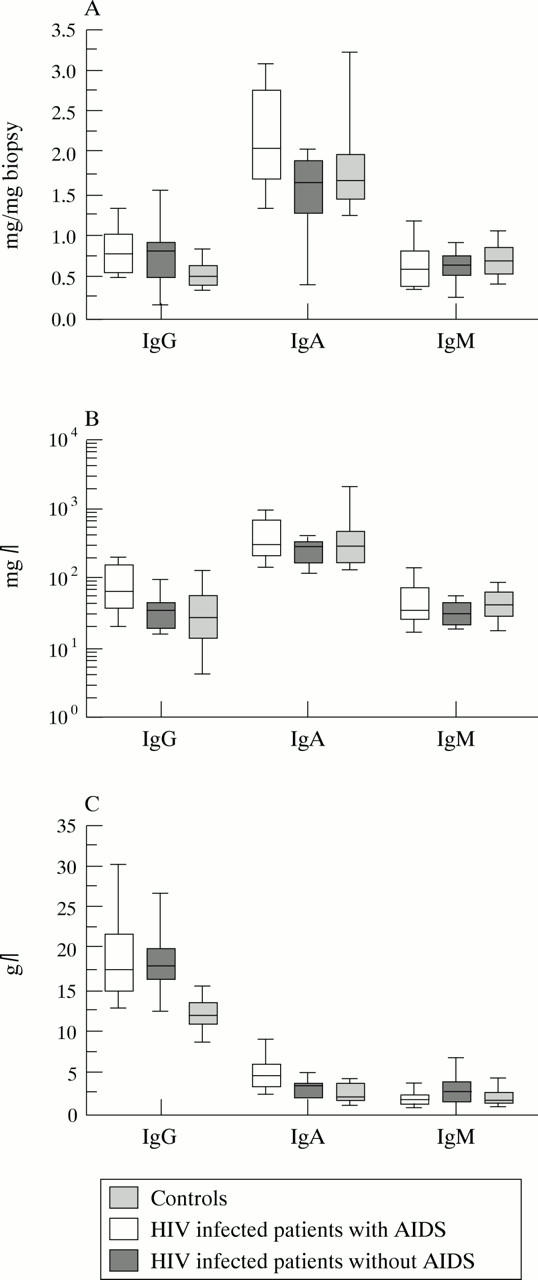
Quantitative analysis of the three isotypes in (A) duodenal biopsy supernatants, (B) saliva, and (C) serum from controls, HIV infected patients with and without AIDS displayed as box plots. Bold horizontal bars represent the median; lower and upper ends of the boxes represent 25th and 75th percentiles respectively; lower and upper "whiskers" represent the 10th and 90th percentiles respectively.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crabtree J. E., Taylor J. D., Wyatt J. I., Heatley R. V., Shallcross T. M., Tompkins D. S., Rathbone B. J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- Eriksson K., Kilander A., Hagberg L., Norkrans G., Holmgren J., Czerkinsky C. Virus-specific antibody production and polyclonal B-cell activation in the intestinal mucosa of HIV-infected individuals. AIDS. 1995 Jul;9(7):695–700. doi: 10.1097/00002030-199507000-00005. [DOI] [PubMed] [Google Scholar]
- Hörnquist C. E., Ekman L., Grdic K. D., Schön K., Lycke N. Y. Paradoxical IgA immunity in CD4-deficient mice. Lack of cholera toxin-specific protective immunity despite normal gut mucosal IgA differentiation. J Immunol. 1995 Sep 15;155(6):2877–2887. [PubMed] [Google Scholar]
- Janoff E. N., Jackson S., Wahl S. M., Thomas K., Peterman J. H., Smith P. D. Intestinal mucosal immunoglobulins during human immunodeficiency virus type 1 infection. J Infect Dis. 1994 Aug;170(2):299–307. doi: 10.1093/infdis/170.2.299. [DOI] [PubMed] [Google Scholar]
- Kotler D. P., Reka S., Clayton F. Intestinal mucosal inflammation associated with human immunodeficiency virus infection. Dig Dis Sci. 1993 Jun;38(6):1119–1127. doi: 10.1007/BF01295730. [DOI] [PubMed] [Google Scholar]
- Kotler D. P., Scholes J. V., Tierney A. R. Intestinal plasma cell alterations in acquired immunodeficiency syndrome. Dig Dis Sci. 1987 Feb;32(2):129–138. doi: 10.1007/BF01297100. [DOI] [PubMed] [Google Scholar]
- Lim S. G., Condez A., Lee C. A., Johnson M. A., Elia C., Poulter L. W. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin Exp Immunol. 1993 Jun;92(3):448–454. doi: 10.1111/j.1365-2249.1993.tb03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Nahm M. H. Expression of human immunoglobulin G subclasses in inflammatory bowel disease. Gastroenterology. 1987 Nov;93(5):1127–1129. doi: 10.1016/0016-5085(87)90578-6. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Stenson W. F. Alterations of the immune system in ulcerative colitis and Crohn's disease. Adv Immunol. 1988;42:285–328. doi: 10.1016/s0065-2776(08)60848-2. [DOI] [PubMed] [Google Scholar]
- Mandel I. D., Barr C. E., Turgeon L. Longitudinal study of parotid saliva in HIV-1 infection. J Oral Pathol Med. 1992 May;21(5):209–213. doi: 10.1111/j.1600-0714.1992.tb00103.x. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Elson C. O., Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989 May;9(3):175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- Müller F., Frøland S. S., Hvatum M., Radl J., Brandtzaeg P. Both IgA subclasses are reduced in parotid saliva from patients with AIDS. Clin Exp Immunol. 1991 Feb;83(2):203–209. doi: 10.1111/j.1365-2249.1991.tb05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Fauci A. S. The effect of cytokines and pharmacologic agents on chronic HIV infection. AIDS Res Hum Retroviruses. 1992 Feb;8(2):191–197. doi: 10.1089/aid.1992.8.191. [DOI] [PubMed] [Google Scholar]
- Quesnel A., Moja P., Lucht F., Touraine J. L., Pozzetto B., Genin C. Is there IgA of gut mucosal origin in the serum of HIV1 infected patients? Gut. 1994 Jun;35(6):803–808. doi: 10.1136/gut.35.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T., Jahn H. U., Schmidt W., Riecken E. O., Zeitz M., Ullrich R. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut. 1995 Oct;37(4):524–529. doi: 10.1136/gut.37.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K., Warnecke C., Brinkmann V., Gelderblom H. R., Bienzle U., Pauli G. Sensitivity of HIV antibody detection in saliva. Med Microbiol Immunol. 1993 Jul;182(3):147–151. doi: 10.1007/BF00190267. [DOI] [PubMed] [Google Scholar]
- Sweet S. P., Rahman D., Challacombe S. J. IgA subclasses in HIV disease: dichotomy between raised levels in serum and decreased secretion rates in saliva. Immunology. 1995 Dec;86(4):556–559. [PMC free article] [PubMed] [Google Scholar]