Abstract
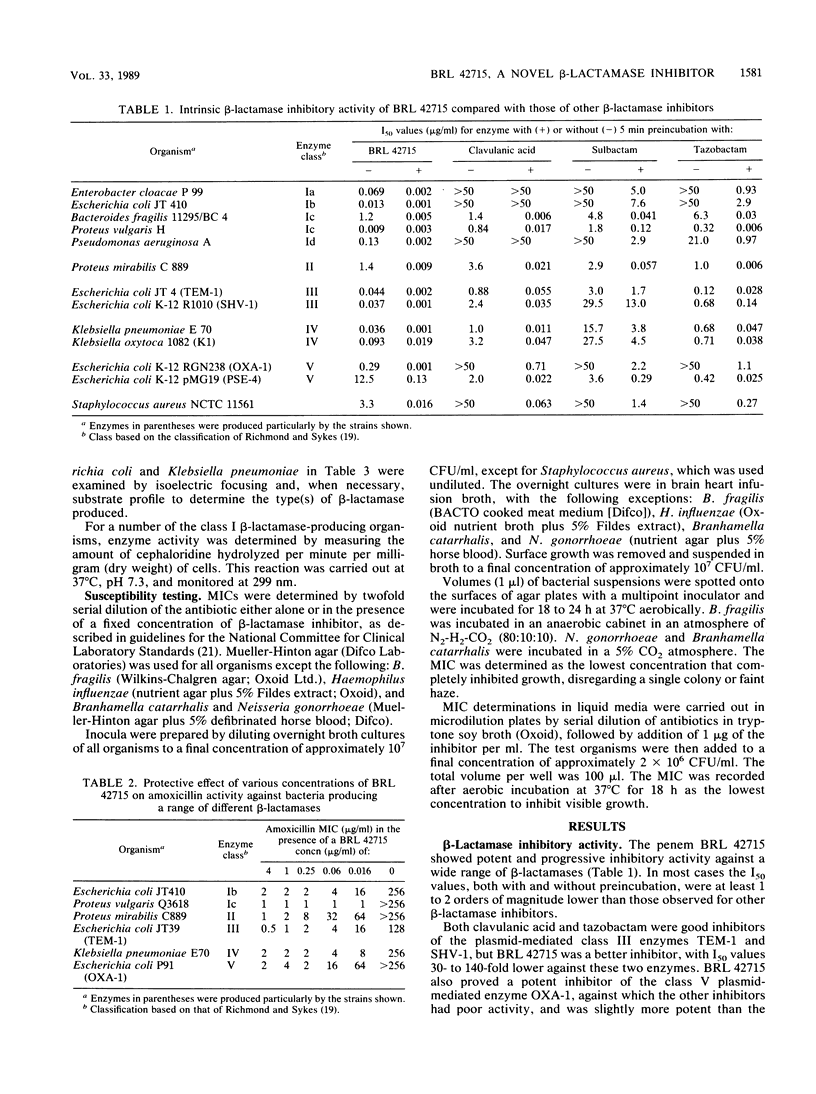
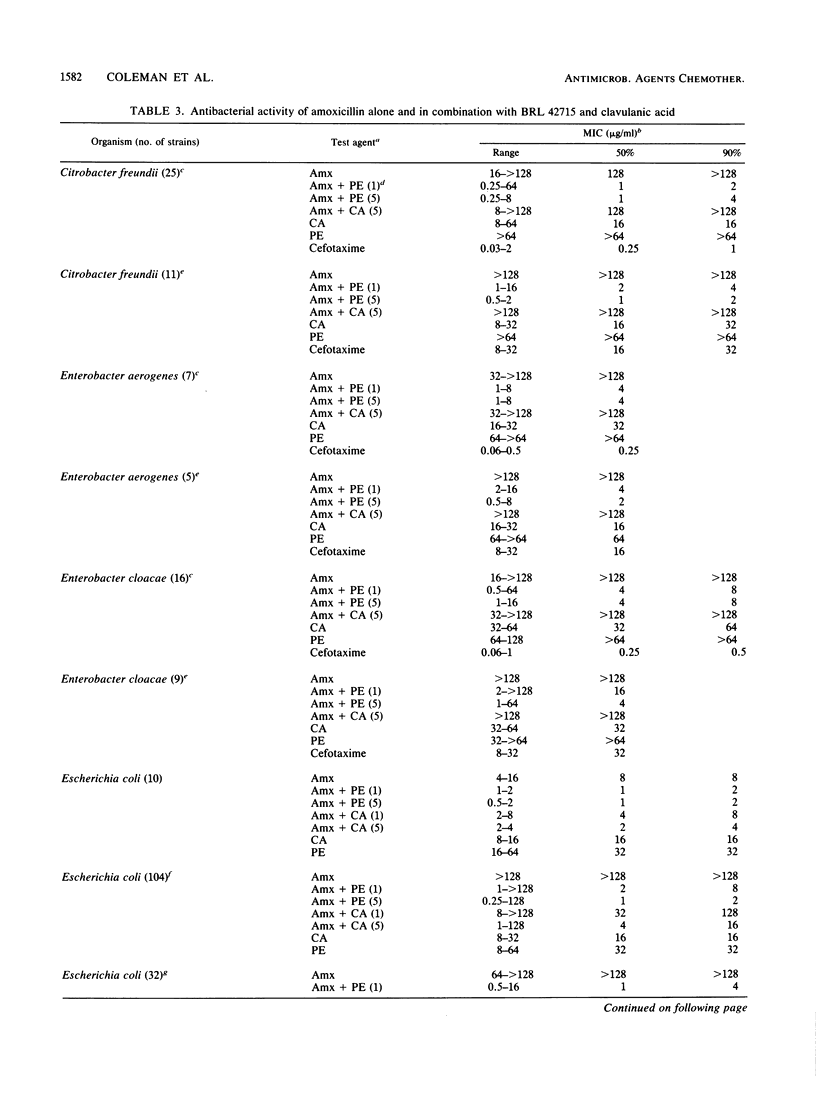
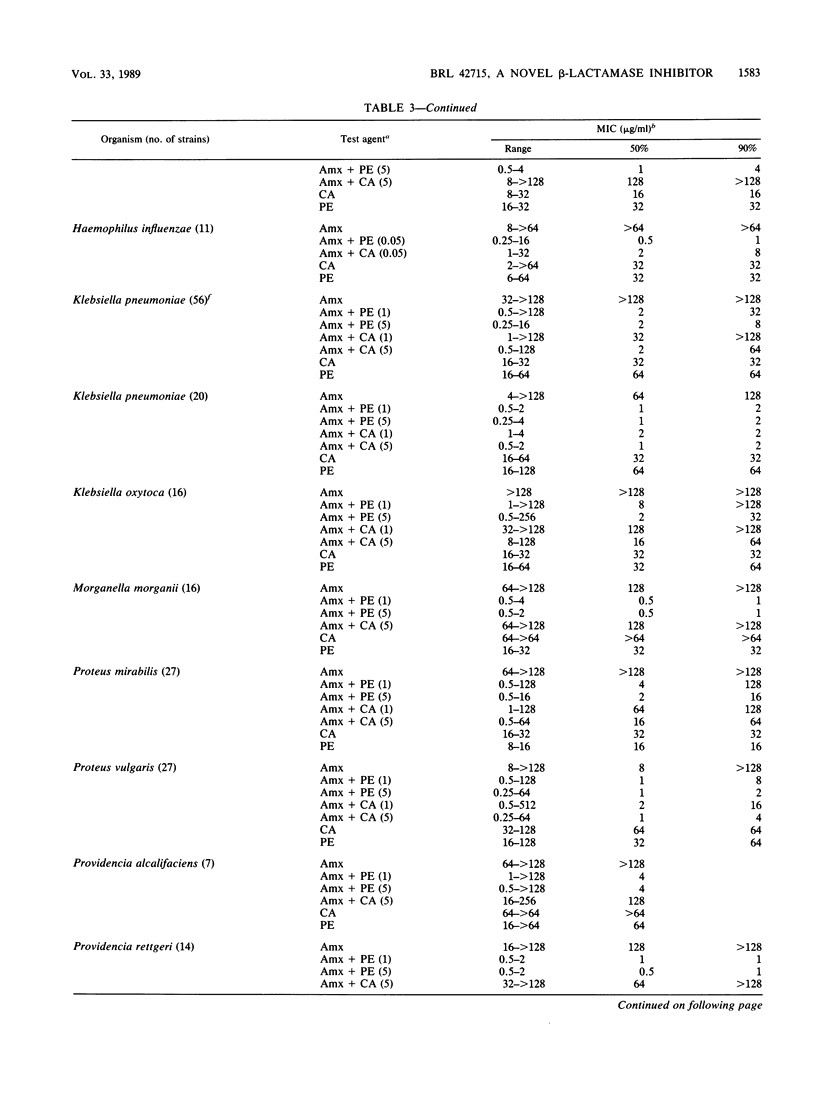
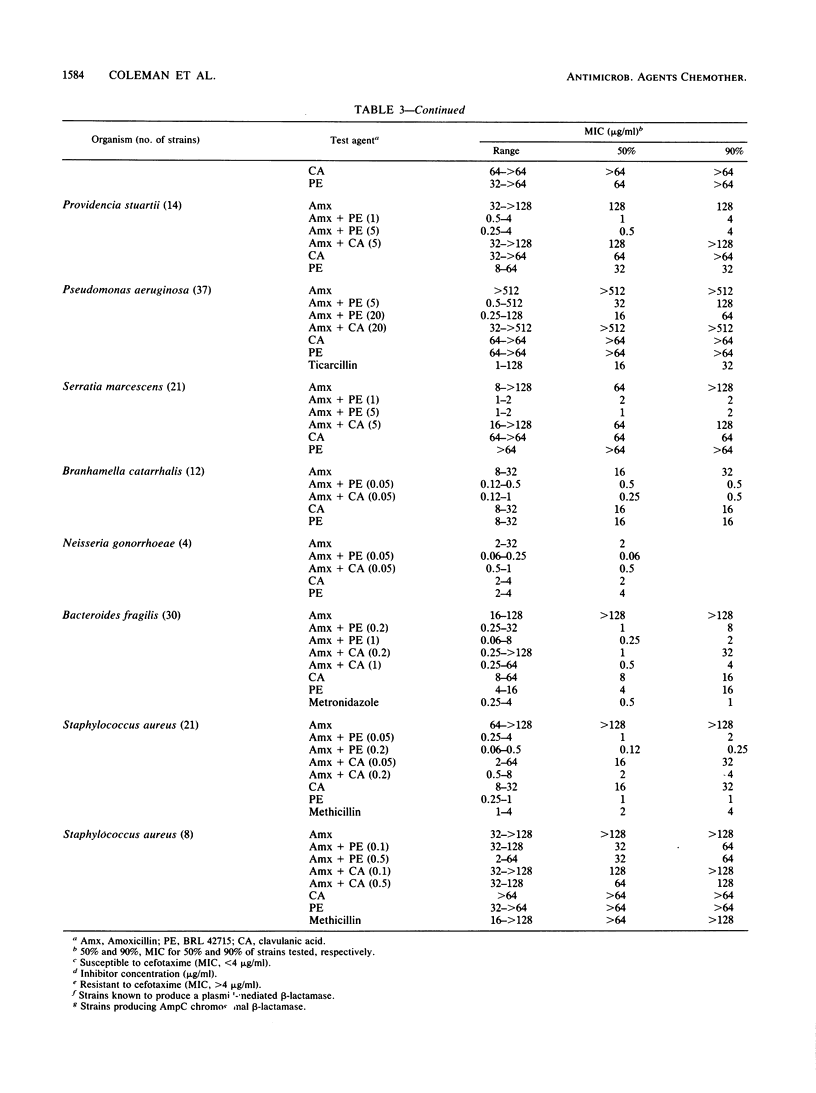
The penem BRL 42715, C6-(N1-methyl-1,2,3-triazolylmethylene)penem, is a potent inhibitor of a broad range of bacterial beta-lactamases, including the plasmid-mediated TEM, SHV, OXA, and staphylococcal enzymes, as well as the chromosomally mediated enzymes of Bacteroides, Enterobacter, Citrobacter, Serratia, Morganella, Escherichia, Klebsiella, and Proteus species. The concentration of BRL 42715 needed to reduce the initial rate of hydrolysis of most beta-lactamase enzymes by 50% was less than 0.01 micrograms/ml, which was 10- to 100-fold lower than for other beta-lactamase inhibitors. These potent inhibitory activities were reflected in the low concentrations of BRL 42715 needed to potentiate the antibacterial activity of beta-lactamase-susceptible beta-lactams. Concentrations of 0.25 micrograms/ml or less considerably enhanced the activity of amoxicillin against many beta-lactamase-producing strains. The MIC50 (MIC for 50% of strains tested) of amoxicillin for 412 beta-lactamase-producing members of the family Enterobacteriaceae fell from greater than 128 to 2 micrograms/ml in the presence of 1 microgram of BRL 42715 per ml, whereas 5 micrograms of clavulanic acid per ml brought the MIC50 down to 8 micrograms/ml. Among these 412 strains were 73 Citrobacter and Enterobacter strains, and 1 microgram of BRL 42715 per ml reduced the MIC50 of amoxicillin from greater than 128 to 2 micrograms/ml for the 48 cefotaxime-susceptible strains and from greater than 128 to 8 micrograms/ml for the 25 cefotaxime-resistant strains.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronoff S. C., Jacobs M. R., Johenning S., Yamabe S. Comparative activities of the beta-lactamase inhibitors YTR 830, sodium clavulanate, and sulbactam combined with amoxicillin or ampicillin. Antimicrob Agents Chemother. 1984 Oct;26(4):580–582. doi: 10.1128/aac.26.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström S., Normark S. Beta-lactam resistance in clinical isolates of Escherichia coli caused by elevated production of the ampC-mediated chromosomal beta-lactamase. Antimicrob Agents Chemother. 1979 Oct;16(4):427–433. doi: 10.1128/aac.16.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Characterization of beta-lactamases. Antimicrob Agents Chemother. 1989 Mar;33(3):259–263. doi: 10.1128/aac.33.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworzack D. L., Pugsley M. P., Sanders C. C., Horowitz E. A. Emergence of resistance in gram-negative bacteria during therapy with expanded-spectrum cephalosporins. Eur J Clin Microbiol. 1987 Aug;6(4):456–459. doi: 10.1007/BF02013110. [DOI] [PubMed] [Google Scholar]
- English A. R., Retsema J. A., Girard A. E., Lynch J. E., Barth W. E. CP-45,899, a beta-lactamase inhibitor that extends the antibacterial spectrum of beta-lactams: initial bacteriological characterization. Antimicrob Agents Chemother. 1978 Sep;14(3):414–419. doi: 10.1128/aac.14.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J. Inconsistency of synergy between the beta-lactamase inhibitor CP-45,899 and beta-lactam antibiotics against multiply drug-resistant Enterobacteriaceae and pseudomonas species. Antimicrob Agents Chemother. 1981 Feb;19(2):361–363. doi: 10.1128/aac.19.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Jackson D. B., Sherris J. C. Development of resistance to cephalosporins in clinical strains of Citrobacter spp. Antimicrob Agents Chemother. 1984 May;25(5):591–595. doi: 10.1128/aac.25.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P. A., Coleman K., Fisher J., Taylor D. In vitro synergistic properties of clavulanic acid, with ampicillin, amoxycillin and ticarcillin. J Antimicrob Chemother. 1980 Jul;6(4):455–470. doi: 10.1093/jac/6.4.455. [DOI] [PubMed] [Google Scholar]
- Jacobs M. R., Aronoff S. C., Johenning S., Shlaes D. M., Yamabe S. Comparative activities of the beta-lactamase inhibitors YTR 830, clavulanate, and sulbactam combined with ampicillin and broad-spectrum penicillins against defined beta-lactamase-producing aerobic gram-negative bacilli. Antimicrob Agents Chemother. 1986 Jun;29(6):980–985. doi: 10.1128/aac.29.6.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W., Smith J. T. Types of beta-lactamase determined by plasmids in gram-negative bacteria. J Bacteriol. 1979 Jun;138(3):657–662. doi: 10.1128/jb.138.3.657-662.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M. Plasmid-mediated beta-lactamases of Gram-negative bacteria: properties and distribution. J Antimicrob Chemother. 1979 Jul;5(4):349–358. doi: 10.1093/jac/5.4.349. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. doi: 10.1128/jb.153.1.281-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara H. Antibiotic resistance in pathogenic and producing bacteria, with special reference to beta-lactam antibiotics. Microbiol Rev. 1981 Dec;45(4):591–619. doi: 10.1128/mr.45.4.591-619.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C., Cole M. Clavulanic acid: a beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother. 1977 May;11(5):852–857. doi: 10.1128/aac.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C., Cole M. Structure-activity relationships amongst beta-lactamase inhibitors. J Enzyme Inhib. 1986;1(2):83–104. doi: 10.3109/14756368609020108. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Sanders C. C. Chromosomal cephalosporinases responsible for multiple resistance to newer beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:573–593. doi: 10.1146/annurev.mi.41.100187.003041. [DOI] [PubMed] [Google Scholar]
- Yang Y. J., Livermore D. M., Williams R. J. Chromosomal beta-lactamase expression and antibiotic resistance in Enterobacter cloacae. J Med Microbiol. 1988 Mar;25(3):227–233. doi: 10.1099/00222615-25-3-227. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J Bacteriol. 1982 Nov;152(2):636–642. doi: 10.1128/jb.152.2.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



