Abstract
Background—The relapse rate after steroid induced remission in Crohn's disease is high. Aims—To test whether oral pH modified release budesonide (3 × 1 mg/day) reduces the relapse rate and to identify patient subgroups with an increased risk of relapse. Methods—In a multicentre, randomised, double blind study, 179 patients with steroid induced remission of Crohn's disease received either 3 × 1 mg budesonide (n=84) or placebo (n=95) for one year. The primary study aim was the maintenance of remission of Crohn's disease for one year. Results—Patient characteristics at study entry were similar for both groups. The relapse rate was 67% (56/84) in the budesonide group and 65% (62/95) in the placebo group. The relapse curves in both groups were similar. The mean time to relapse was 93.5days in the budesonide group and 67.0 days in the placebo group. No prognostic factors allowing prediction of an increased risk for relapse or definition of patient subgroups who derived benefit from low dose budesonide were found. Drug related side effects were mild and no different between the budesonide and the placebo group. Conclusion—Oral pH modified release budesonide at a dose of 3 × 1 mg/day is not effective for maintaining steroid induced remission in Crohn's disease.
Keywords: budesonide; Crohn's disease; maintenance of remission
Full Text
The Full Text of this article is available as a PDF (106.8 KB).
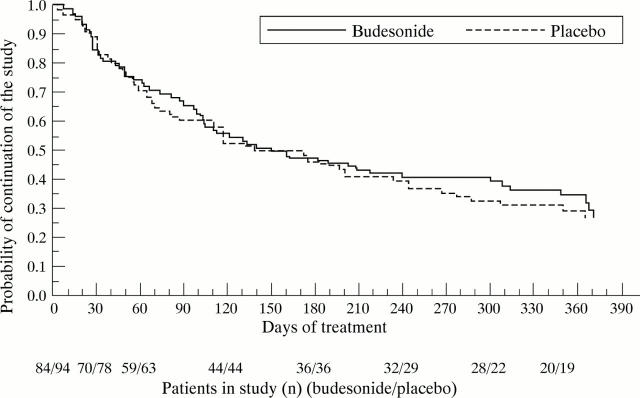
Figure 1 .
Time to relapse after randomisation and start of maintenance treatment.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Candy S., Wright J., Gerber M., Adams G., Gerig M., Goodman R. A controlled double blind study of azathioprine in the management of Crohn's disease. Gut. 1995 Nov;37(5):674–678. doi: 10.1136/gut.37.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewe K., Press A. G., Singe C. C., Stufler M., Ueberschaer B., Hommel G., Meyer zum Büschenfelde K. H. Azathioprine combined with prednisolone or monotherapy with prednisolone in active Crohn's disease. Gastroenterology. 1993 Aug;105(2):367–372. doi: 10.1016/0016-5085(93)90709-l. [DOI] [PubMed] [Google Scholar]
- Gendre J. P., Mary J. Y., Florent C., Modigliani R., Colombel J. F., Soulé J. C., Galmiche J. P., Lerebours E., Descos L., Viteau J. M. Oral mesalamine (Pentasa) as maintenance treatment in Crohn's disease: a multicenter placebo-controlled study. The Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID) Gastroenterology. 1993 Feb;104(2):435–439. doi: 10.1016/0016-5085(93)90411-5. [DOI] [PubMed] [Google Scholar]
- Greenberg G. R., Feagan B. G., Martin F., Sutherland L. R., Thomson A. B., Williams C. N., Nilsson L. G., Persson T. Oral budesonide as maintenance treatment for Crohn's disease: a placebo-controlled, dose-ranging study. Canadian Inflammatory Bowel Disease Study Group. Gastroenterology. 1996 Jan;110(1):45–51. doi: 10.1053/gast.1996.v110.pm8536887. [DOI] [PubMed] [Google Scholar]
- Greenberg G. R., Feagan B. G., Martin F., Sutherland L. R., Thomson A. B., Williams C. N., Nilsson L. G., Persson T. Oral budesonide for active Crohn's disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med. 1994 Sep 29;331(13):836–841. doi: 10.1056/NEJM199409293311303. [DOI] [PubMed] [Google Scholar]
- Gross V., Andus T., Caesar I., Bischoff S. C., Lochs H., Tromm A., Schulz H. J., Bär U., Weber A., Gierend M. Oral pH-modified release budesonide versus 6-methylprednisolone in active Crohn's disease. German/Austrian Budesonide Study Group. Eur J Gastroenterol Hepatol. 1996 Sep;8(9):905–909. [PubMed] [Google Scholar]
- Landi B., Anh T. N., Cortot A., Soule J. C., Rene E., Gendre J. P., Bories P., See A., Metman E. H., Florent C. Endoscopic monitoring of Crohn's disease treatment: a prospective, randomized clinical trial. The Groupe d'Etudes Therapeutiques des Affections Inflammatoires Digestives. Gastroenterology. 1992 May;102(5):1647–1653. doi: 10.1016/0016-5085(92)91725-j. [DOI] [PubMed] [Google Scholar]
- Lochs H., Steinhardt H. J., Klaus-Wentz B., Zeitz M., Vogelsang H., Sommer H., Fleig W. E., Bauer P., Schirrmeister J., Malchow H. Comparison of enteral nutrition and drug treatment in active Crohn's disease. Results of the European Cooperative Crohn's Disease Study. IV. Gastroenterology. 1991 Oct;101(4):881–888. doi: 10.1016/0016-5085(91)90711-s. [DOI] [PubMed] [Google Scholar]
- Löfberg R., Danielsson A., Salde L. Oral budesonide in active Crohn's disease. Aliment Pharmacol Ther. 1993 Dec;7(6):611–616. doi: 10.1111/j.1365-2036.1993.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Löfberg R., Rutgeerts P., Malchow H., Lamers C., Danielsson A., Olaison G., Jewell D., Ostergaard Thomsen O., Lorenz-Meyer H., Goebell H. Budesonide prolongs time to relapse in ileal and ileocaecal Crohn's disease. A placebo controlled one year study. Gut. 1996 Jul;39(1):82–86. doi: 10.1136/gut.39.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow H., Ewe K., Brandes J. W., Goebell H., Ehms H., Sommer H., Jesdinsky H. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984 Feb;86(2):249–266. [PubMed] [Google Scholar]
- Modigliani R., Colombel J. F., Dupas J. L., Dapoigny M., Costil V., Veyrac M., Duclos B., Soulé J. C., Gendre J. P., Galmiche J. P. Mesalamine in Crohn's disease with steroid-induced remission: effect on steroid withdrawal and remission maintenance, Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gastroenterology. 1996 Mar;110(3):688–693. doi: 10.1053/gast.1996.v110.pm8608877. [DOI] [PubMed] [Google Scholar]
- Pearson D. C., May G. R., Fick G. H., Sutherland L. R. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995 Jul 15;123(2):132–142. doi: 10.7326/0003-4819-123-2-199507150-00009. [DOI] [PubMed] [Google Scholar]
- Present D. H., Korelitz B. I., Wisch N., Glass J. L., Sachar D. B., Pasternack B. S. Treatment of Crohn's disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980 May 1;302(18):981–987. doi: 10.1056/NEJM198005013021801. [DOI] [PubMed] [Google Scholar]
- Roth M., Gross V., Schölmerich J., Ueberschaer B., Ewe K. Treatment of active Crohn's disease with an oral slow-release budesonide formulation. Am J Gastroenterol. 1993 Jun;88(6):968–969. [PubMed] [Google Scholar]
- Rutgeerts P., Löfberg R., Malchow H., Lamers C., Olaison G., Jewell D., Danielsson A., Goebell H., Thomsen O. O., Lorenz-Meyer H. A comparison of budesonide with prednisolone for active Crohn's disease. N Engl J Med. 1994 Sep 29;331(13):842–845. doi: 10.1056/NEJM199409293311304. [DOI] [PubMed] [Google Scholar]
- Steinhart A. H., Hemphill D., Greenberg G. R. Sulfasalazine and mesalazine for the maintenance therapy of Crohn's disease: a meta-analysis. Am J Gastroenterol. 1994 Dec;89(12):2116–2124. [PubMed] [Google Scholar]
- Summers R. W., Switz D. M., Sessions J. T., Jr, Becktel J. M., Best W. R., Kern F., Jr, Singleton J. W. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology. 1979 Oct;77(4 Pt 2):847–869. [PubMed] [Google Scholar]