Abstract
Background—Expression of pro-inflammatory cytokines is increased in the intestinal lamina propria of patients with inflammatory bowel disease (IBD). Nuclear factor κB (NFκB) controls transcription of inflammation genes. On activation, NFκB is rapidly released from its cytoplasmic inhibitor (IκB), transmigrates into the nucleus, and binds to DNA response elements in gene promoter regions. Aims—To investigate whether increased activation of NFκB is important in IBD and may be down-regulated by anti-inflammatory treatment. Methods—Activation of NFκB was determined by western blot assessment and electrophoretic mobility shift assay in nuclear extracts of colonic biopsy samples as well as lamina propria mononuclear cells. Results—Nuclear levels of NFκB p65 are increased in lamina propria biopsy specimens from patients with Crohn's disease in comparison with patients with ulcerative colitis and controls. Increased activation of NFκB was detected in lamina propria mononuclear cells from patients with active IBD. Corticosteroids strongly inhibit intestinal NFκB activation in IBD in vivo and in vitro by stabilising the cytosolic inhibitor IκBα against activation induced degradation. Conclusions—In both IBDs, but particularly Crohn's disease, increased activation of NFκB may be involved in the regulation of the inflammatory response. Inhibition of NFκB activation may represent a mechanism by which steroids exert an anti-inflammatory effect in IBD.
Keywords: interleukin 1ß; inflammatory bowel disease; intestinal immunity; signal transduction; steroids; tumour necrosis factor α
Full Text
The Full Text of this article is available as a PDF (204.3 KB).
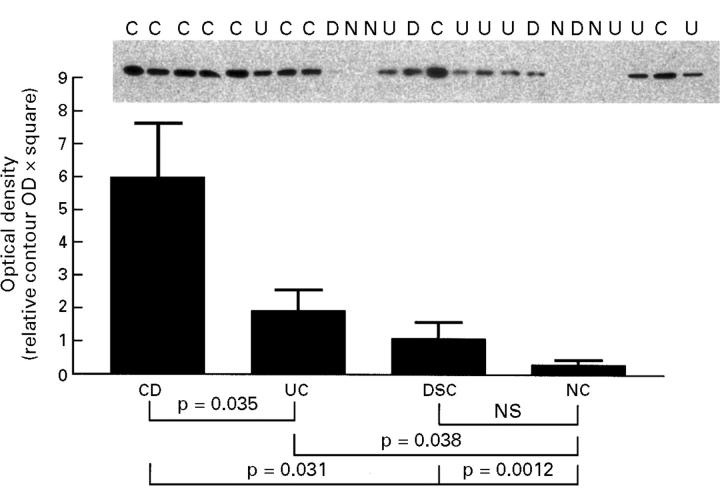
Figure 1 .
NFκB p65 in nuclear extracts from colonic biospsy samples. Colonic biopsy specimens from 10 patients with active Crohn's disease (CD), 14 patients with active ulcerative colitis (UC), six disease specificity controls (DSC), and seven normal controls (NC) were extracted using a procedure to isolate the nuclear compartment. Densitometric readings from western blot assessments of NFκB p65 are demonstrated by the bar graph (mean (SD)). Before electrophoresis, samples were adjusted to have equal contents of total protein. The inset shows a representative sample of the original blots. Individuals are marked on the blot with U (ulcerative colitis), C (Crohn's disease), D (disease specificity controls), or N (normal volunteer). The characteristics of the patients and localisation of the biopsy samples are shown in table 1. The disease specificity control patients shown in the blot are patients 28, 25, 27, and 29 (from left to right).
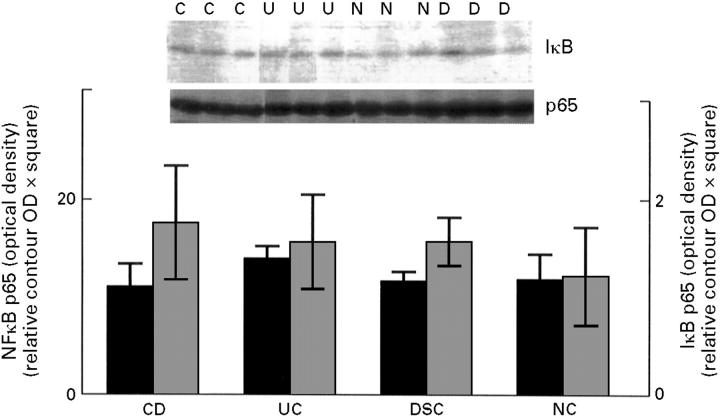
Figure 2 .
Total NFκB p65 and IκBα in colonic biospy tissue. Colonic biopsy specimens were homogenised and analysed by western blot without prior compartmentalised extraction. Densitometric readings are shown by the bar graph (mean (SD)). The filled bars show levels of total NFκB p65 (left y axis), and the shaded bars levels of IκBα (right y axis). Before electrophoresis, samples were adjusted to have equal contents of total protein. No statistical differences were seen between patients with active Crohn's disease (CD, n = 6), patients with active ulcerative colitis (UC, n = 6), disease specificity controls (DSC, n = 6), and normal controls (NC, n = 5). The inset shows representative blots (C = Crohn's disease, U = ulcerative colitis, D = disease specificity control, and N = normal control). Biopsy specimens were taken from the same location as those used for nuclear extracts (fig 1).
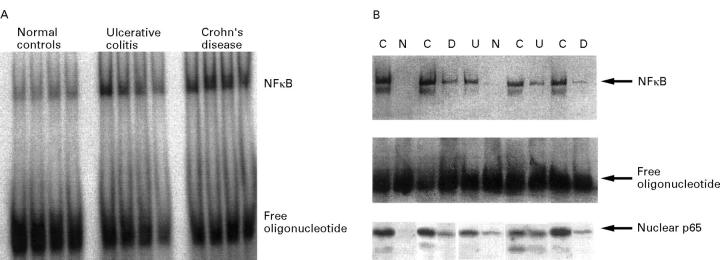
Figure 3 .
Detection of NFκB in the intestinal lamina propria. (A) Nuclear extracts were prepared from colonoscopic biopsy specimens. A representative radioactive electrophoretic mobility shift assay using consensus oligonucleotides to detect NFκB in nuclear extracts from intestinal tissue is shown. In both Crohn's disease (n = 9) and ulcerative colitis (n = 8) increased levels of NFκB were found in comparison with disease specificity controls (n = 5) and healthy volunteers (n = 8). (B) Nuclear extracts were prepared from LPMNCs, which were freshly isolated from colonic biopsy specimens. A blot from a representative non-radioactive gel shift experiment with ten samples (C = Crohn's disease; U = ulcerative colitis; D = disease specificity controls; N = normal controls) is shown. The highest levels of activated NFκB were seen in patients with active Crohn's disease, with similar levels in all four samples. In only one of four normal control samples could low levels of oligonucleotide binding proteins be detected. Levels in patients with ulcerative colitis (four) as well as disease specificity controls (three) appeared to be increased as well, although not as much as in Crohn's disease. The presence of NFκB p65 as part of the complex was controlled by supershift experiments (not shown). The levels of nuclear p65 determined in the same extracts by western blot are shown in the lower part of the figure.
Figure 4 .
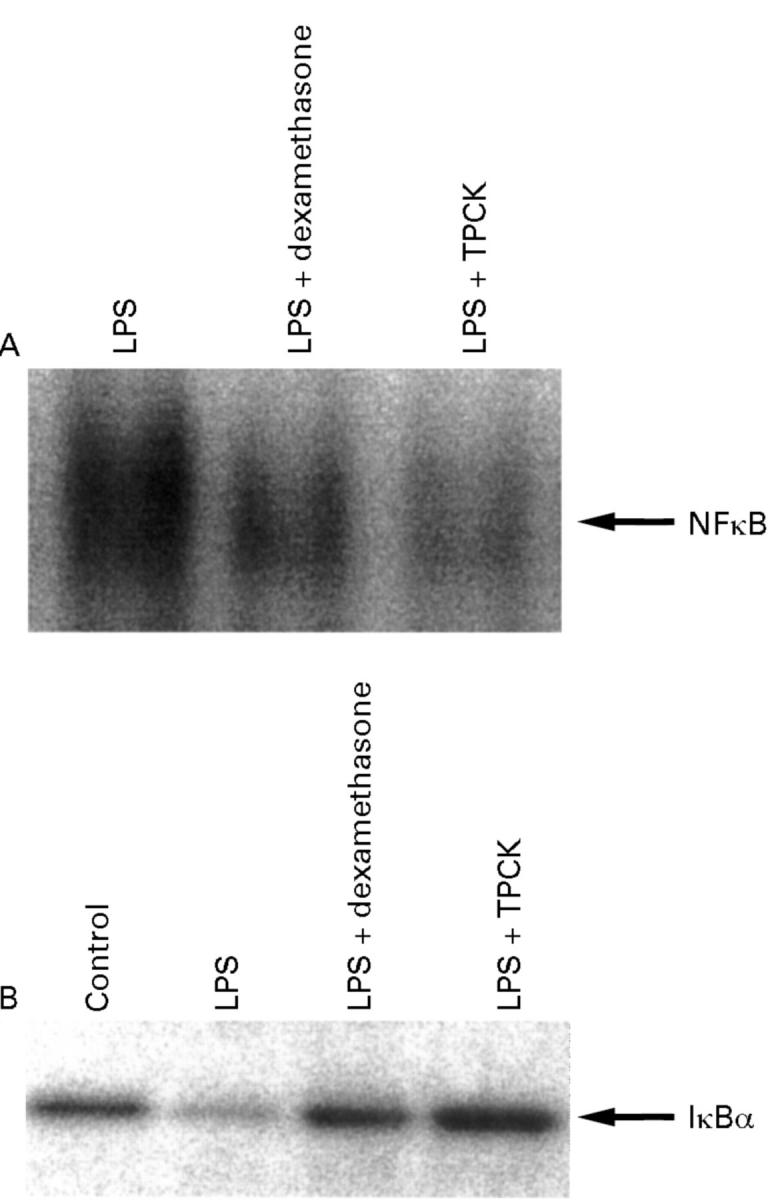
Inhibition of NFκB activation (A) and IκBα degradation (B) by steroids (dexamethasone) and N-α-tosyl-phenylalanine chloromethyl ketone (TPCK). Nuclear extracts were prepared from lamnia propria mononuclear cells obtained from colonoscopic biopsy samples (two patients with Crohn's disease and two normal volunteer controls). Cells were cultured for 30 minutes in the presence of lipopolysaccharide (LPS). A representative blot from a patient with Crohn's disease is shown. (A) Both dexamethasone (10 µM) and TPCK (30 µM) strongly reduced the amounts of NFκB available for binding to consensus oligonucleotides. (B) IκBα was assessed in total extracts by western blot. TPCK as well as dexamethasone stabilised IκBα against activation induced degradation.
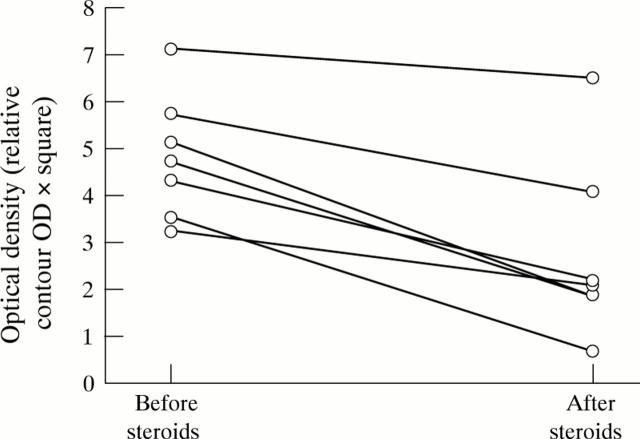
Figure 5 .
Nuclear NFκB p65 from colonoscopic biopsy specimens from patients with inflammatory bowel disease is decreased by steroid treatment. Sigmoid biopsy specimens were obtained from patients with moderately to highly active ileocolonic Crohn's disease and involvement of the sigmoid (CDAI 250-450). Nuclear extracts were prepared and concentrations of NFκB p65 were assessed by western blot. Seven days of treatment with steroids (60 mg prednisolone/day per os in addition to 5 mg betamethasone twice daily as an enema) induced a significant reduction (p = 0.0156) in nuclear NFκB p65 concentration. IκBα levels remained unchanged (data not shown).
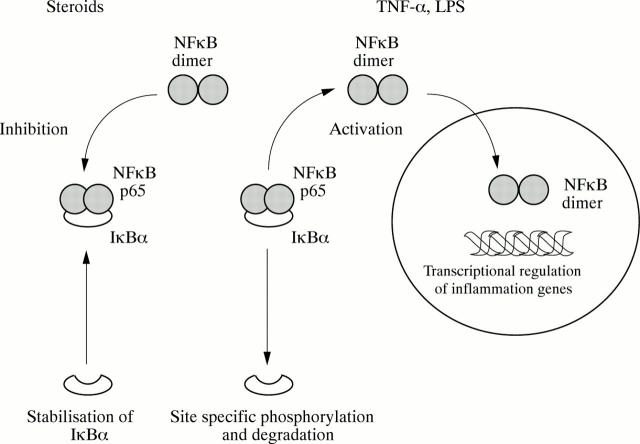
Figure 6 .
Schematic representation of NFκB activation and inhibition by steroids. Activating stimuli including tumour necrosis factor α (TNF-α) and lipopolysaccharide (LPS) promote degradation of IκBα and the subsequent release of NFκB p65 into the cytosolic compartment. NFκB p65 translocates into the nucleus and forms dimers as part of the activation process. Functionally active NFκB can then bind to specific sites in inflammation gene promoter regions and initiate transcription. Steroids appear to stabilise IκBα against activation induced degradation and thereby reduce the amount of functionally active NFκB available in the nucleus.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auphan N., DiDonato J. A., Rosette C., Helmberg A., Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995 Oct 13;270(5234):286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Brown K., Gerstberger S., Carlson L., Franzoso G., Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995 Mar 10;267(5203):1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- Dent C. L., Latchman D. S. The overlapping octamer/TAATGARAT motif is a high-affinity binding site for the cellular transcription factors Oct-1 and Oct-2. Biochem J. 1991 Jul 15;277(Pt 2):541–545. doi: 10.1042/bj2770541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M. G. Measurement of protein-DNA interaction parameters by electrophoresis mobility shift assay. Electrophoresis. 1989 May-Jun;10(5-6):366–376. doi: 10.1002/elps.1150100515. [DOI] [PubMed] [Google Scholar]
- Hassanain H. H., Dai W., Gupta S. L. Enhanced gel mobility shift assay for DNA-binding factors. Anal Biochem. 1993 Aug 15;213(1):162–167. doi: 10.1006/abio.1993.1400. [DOI] [PubMed] [Google Scholar]
- Henell F., Glaumann H. Effect of leupeptin on the autophagic vacuolar system of rat hepatocytes. Correlation between ultrastructure and degradation of membrane and cytosolic proteins. Lab Invest. 1984 Jul;51(1):46–56. [PubMed] [Google Scholar]
- Hou J., Schindler U., Henzel W. J., Ho T. C., Brasseur M., McKnight S. L. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994 Sep 16;265(5179):1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- Kim H., Lee H. S., Chang K. T., Ko T. H., Baek K. J., Kwon N. S. Chloromethyl ketones block induction of nitric oxide synthase in murine macrophages by preventing activation of nuclear factor-kappa B. J Immunol. 1995 May 1;154(9):4741–4748. [PubMed] [Google Scholar]
- Kopp E., Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994 Aug 12;265(5174):956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Kunsch C., Rosen C. A. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993 Oct;13(10):6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ledebur H. C., Parks T. P. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J Biol Chem. 1995 Jan 13;270(2):933–943. doi: 10.1074/jbc.270.2.933. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Ligumsky M., Simon P. L., Karmeli F., Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990 Jun;31(6):686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Hutchings P., Choy M. Y., Murch S., Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990 Aug;81(2):301–305. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K., Jewell D. P. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989 Jun;30(6):835–838. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Pettersson S., Meyer zum Büschenfelde K. H., Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996 Sep;2(9):998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Nicholls S., Stephens S., Braegger C. P., Walker-Smith J. A., MacDonald T. T. Cytokines in stools of children with inflammatory bowel disease or infective diarrhoea. J Clin Pathol. 1993 Aug;46(8):757–760. doi: 10.1136/jcp.46.8.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989 Jan 14;298(6666):82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecker H. C., Steffen M., Witthoeft T., Pflueger I., Schreiber S., MacDermott R. P., Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993 Oct;94(1):174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohringer R., Holden D. W. Protein blotting: detection of proteins with colloidal gold, and of glycoproteins and lectins with biotin-conjugated and enzyme probes. Anal Biochem. 1985 Jan;144(1):118–127. doi: 10.1016/0003-2697(85)90092-2. [DOI] [PubMed] [Google Scholar]
- Scheinman R. I., Cogswell P. C., Lofquist A. K., Baldwin A. S., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995 Oct 13;270(5234):283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- Scherer D. C., Brockman J. A., Chen Z., Maniatis T., Ballard D. W. Signal-induced degradation of I kappa B alpha requires site-specific ubiquitination. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtz B., Lamb K., Rosfjord E., Kingsley M., Rizzino A. Appearance of nuclear protease activity after embryonal carcinoma cells undergo differentiation. Dev Biol. 1996 Feb 1;173(2):420–427. doi: 10.1006/dbio.1996.0037. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Heinig T., Panzer U., Reinking R., Bouchard A., Stahl P. D., Raedler A. Impaired response of activated mononuclear phagocytes to interleukin 4 in inflammatory bowel disease. Gastroenterology. 1995 Jan;108(1):21–33. doi: 10.1016/0016-5085(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Schreiber S., MacDermott R. P., Raedler A., Pinnau R., Bertovich M. J., Nash G. S. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991 Oct;101(4):1020–1030. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- Shakhov A. N., Collart M. A., Vassalli P., Nedospasov S. A., Jongeneel C. V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990 Jan 1;171(1):35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Walz G., Singaram C., Lipman M. L., Zanker B., Muggia A., Antonioli D., Peppercorn M. A., Strom T. B. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992 Jun;37(6):818–826. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- Traenckner E. B., Wilk S., Baeuerle P. A. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. EMBO J. 1994 Nov 15;13(22):5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Sternsdorf T., Liese J., Belohradsky B., Weber C., Wedel A., Schreck R., Bäuerle P., Ströbel M. Pyrrolidine dithiocarbamate inhibits NF-kappa B mobilization and TNF production in human monocytes. J Immunol. 1993 Dec 15;151(12):6986–6993. [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Wedel A., Schraut W., Ströbel M., Wendelgass P., Sternsdorf T., Bäuerle P. A., Haas J. G., Riethmüller G. Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J Biol Chem. 1994 Jun 24;269(25):17001–17004. [PubMed] [Google Scholar]
- van Dullemen H. M., van Deventer S. J., Hommes D. W., Bijl H. A., Jansen J., Tytgat G. N., Woody J. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology. 1995 Jul;109(1):129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]