Abstract
Background—Concentrations of pro-inflammatory cytokines are increased in the intestinal mucosa of patients with active inflammatory bowel disease (IBD). Polymorphonuclear neutrophil granulocytes (PMN) are the most abundant cell type in intestinal lesions in IBD. Interleukin 10 (IL-10) is an important contra-inflammatory cytokine which induces downregulation of pro-inflammatory cytokines. Aims—To investigate whether PMN from patients with IBD or infectious colitis, respectively, secrete increased amounts of pro-inflammatory cytokines and can be regulated by IL-10. Methods—Secretion (ELISA) as well as corresponding mRNA levels (semiquantitative RT-PCR) of pro-inflammatory cytokines (IL-1β, TNF-α) and of IL-1 receptor antagonist were assessed in peripheral PMN. Results—PMN from patients with IBD are primed to secrete enhanced amounts of pro-inflammatory cytokines accompanied by detection of corresponding mRNAs in comparison with normal controls. This finding is not specific for IBD but rather reflects intestinal inflammation in general. IL-10 markedly inhibited pro-inflammatory cytokine secretion as well as corresponding mRNA concentrations. Conclusions—PMN are an important source of pro-inflammatory cytokines in patients with intestinal inflammation and can be downregulated by IL-10.
Keywords: granulocytes; interleukin 1β; interleukin 10; inflammatory bowel disease; intestinal immunity; inflammation; neutrophils; tumour necrosis factor α
Full Text
The Full Text of this article is available as a PDF (160.7 KB).
Figure 1 .
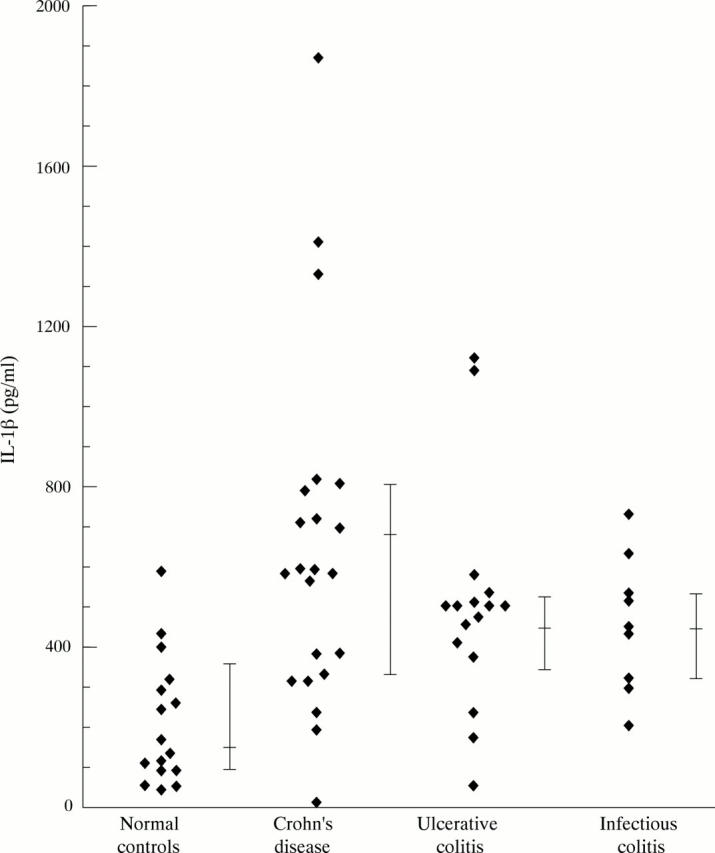
Lipopolysaccharide induced secretion of IL-1β by peripheral blood PMN.
Figure 2 .
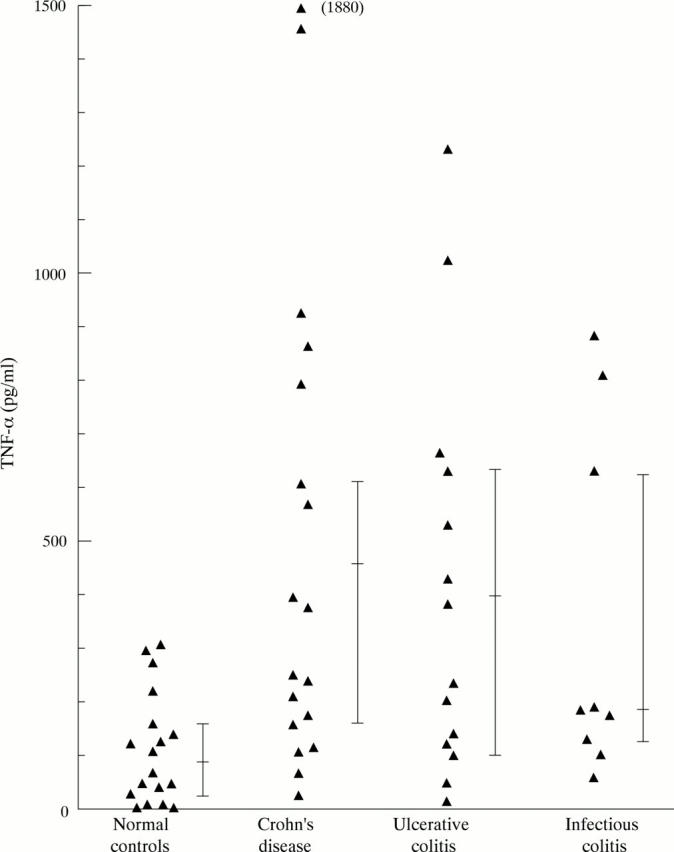
Lipopolysaccharide induced secretion of TNF-α by peripheral blood PMN.
Figure 3 .
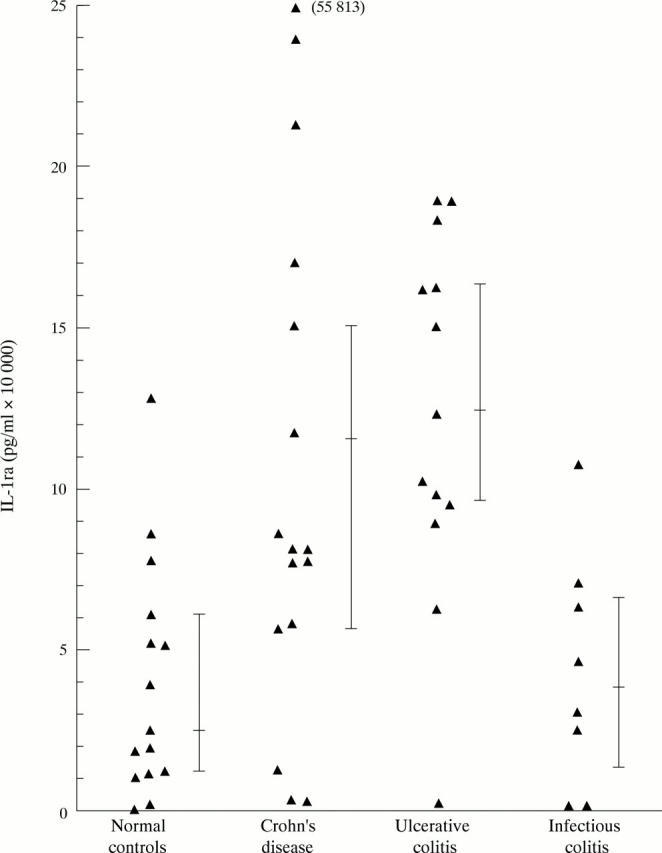
Lipopolysaccharide induced secretion of IL-1ra by peripheral blood PMN.
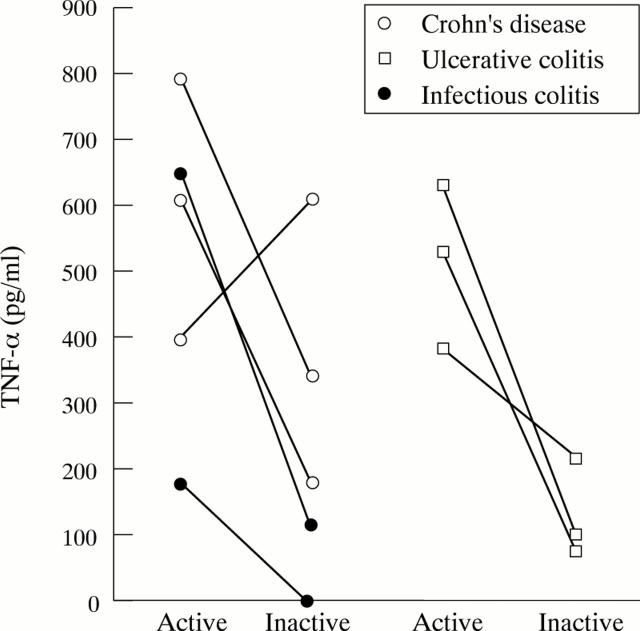
Figure 4 .
Longitudinal follow up of patients with mucosal inflammation.
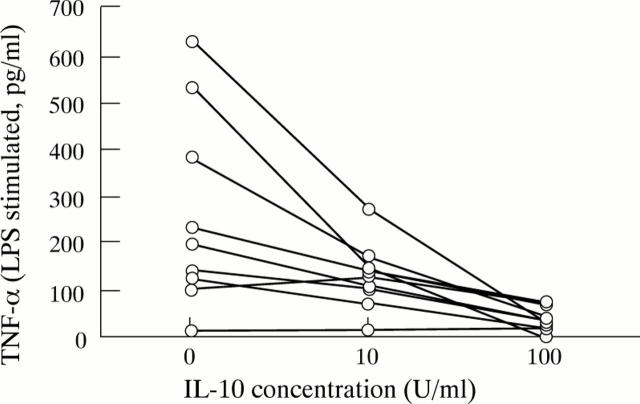
Figure 5 .
Downregulation of PMN pro-inflammatory cytokine secretion by IL-10.
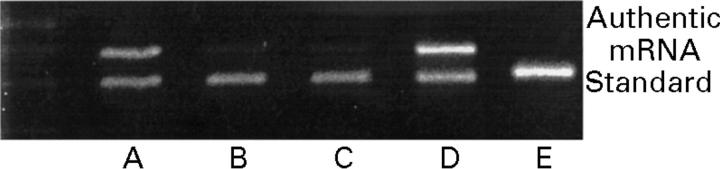
Figure 6 .
Pro-inflammatory cytokine mRNA in PMN and regulation by IL-10.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeyemi E. O., Hodgson H. J. Faecal elastase reflects disease activity in active ulcerative colitis. Scand J Gastroenterol. 1992;27(2):139–142. doi: 10.3109/00365529209165434. [DOI] [PubMed] [Google Scholar]
- Albelda S. M., Smith C. W., Ward P. A. Adhesion molecules and inflammatory injury. FASEB J. 1994 May;8(8):504–512. [PubMed] [Google Scholar]
- Andus T., Gross V., Caesar I., Krumm D., Hosp J., Gerok W., Schölmerich J. PMN-elastase in assessment of patients with inflammatory bowel disease. Dig Dis Sci. 1993 Sep;38(9):1638–1644. doi: 10.1007/BF01303172. [DOI] [PubMed] [Google Scholar]
- Anton P. A., Targan S. R., Shanahan F. Increased neutrophil receptors for and response to the proinflammatory bacterial peptide formyl-methionyl-leucyl-phenylalanine in Crohn's disease. Gastroenterology. 1989 Jul;97(1):20–28. doi: 10.1016/0016-5085(89)91410-8. [DOI] [PubMed] [Google Scholar]
- Baldassano R. N., Schreiber S., Johnston R. B., Jr, Fu R. D., Muraki T., MacDermott R. P. Crohn's disease monocytes are primed for accentuated release of toxic oxygen metabolites. Gastroenterology. 1993 Jul;105(1):60–66. doi: 10.1016/0016-5085(93)90010-a. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Bazzoni F., Cassatella M. A., Laudanna C., Rossi F. Phagocytosis of opsonized yeast induces tumor necrosis factor-alpha mRNA accumulation and protein release by human polymorphonuclear leukocytes. J Leukoc Biol. 1991 Sep;50(3):223–228. doi: 10.1002/jlb.50.3.223. [DOI] [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Bienvenu J., Coulon L., Doche C., Gutowski M. C., Grau G. E. Analytical performances of commercial ELISA-kits for IL-2, IL-6 and TNF-alpha. A WHO study. Eur Cytokine Netw. 1993 Nov-Dec;4(6):447–451. [PubMed] [Google Scholar]
- Brynskov J., Tvede N., Andersen C. B., Vilien M. Increased concentrations of interleukin 1 beta, interleukin-2, and soluble interleukin-2 receptors in endoscopical mucosal biopsy specimens with active inflammatory bowel disease. Gut. 1992 Jan;33(1):55–58. doi: 10.1136/gut.33.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella M. A., Meda L., Bonora S., Ceska M., Constantin G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993 Dec 1;178(6):2207–2211. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella M. A., Meda L., Gasperini S., Calzetti F., Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994 May 1;179(5):1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella M. A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995 Jan;16(1):21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colotta F., Re F., Polentarutti N., Sozzani S., Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992 Oct 15;80(8):2012–2020. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan 14;328(2):106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Faden H., Rossi T. M. Chemiluminescent response of neutrophils from patients with inflammatory bowel disease. Dig Dis Sci. 1985 Feb;30(2):139–142. doi: 10.1007/BF01308200. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Gasperini S., Donini M., Dusi S., Cassatella M. A. Interleukin-10 decreases tyrosine phosphorylation of discrete lipopolysaccharide-induced phosphoproteins in human granulocytes. Biochem Biophys Res Commun. 1995 Apr 6;209(1):87–94. doi: 10.1006/bbrc.1995.1474. [DOI] [PubMed] [Google Scholar]
- Griga T., Tromm A., Schwegler U., May B. Enhanced superoxide anion release of normal neutrophil granulocytes primed with sera of patients with inactive inflammatory bowel disease. Z Gastroenterol. 1995 Jul;33(6):345–348. [PubMed] [Google Scholar]
- Grisham M. B., Granger D. N. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988 Mar;33(3 Suppl):6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- Kelleher D., Feighery C., Weir D. G. Chemiluminescence by polymorphonuclear leucocyte subpopulations in chronic inflammatory bowel disease. Influence of the cell separation procedure. Digestion. 1990;45(3):158–165. doi: 10.1159/000200238. [DOI] [PubMed] [Google Scholar]
- Koizumi M., King N., Lobb R., Benjamin C., Podolsky D. K. Expression of vascular adhesion molecules in inflammatory bowel disease. Gastroenterology. 1992 Sep;103(3):840–847. doi: 10.1016/0016-5085(92)90015-q. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Ligumsky M., Simon P. L., Karmeli F., Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990 Jun;31(6):686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Hutchings P., Choy M. Y., Murch S., Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990 Aug;81(2):301–305. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K., Jewell D. P. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989 Jun;30(6):835–838. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malizia G., Calabrese A., Cottone M., Raimondo M., Trejdosiewicz L. K., Smart C. J., Oliva L., Pagliaro L. Expression of leukocyte adhesion molecules by mucosal mononuclear phagocytes in inflammatory bowel disease. Gastroenterology. 1991 Jan;100(1):150–159. doi: 10.1016/0016-5085(91)90595-c. [DOI] [PubMed] [Google Scholar]
- Monajemi H., Meenan J., Lamping R., Obradov D. O., Radema S. A., Trown P. W., Tytgat G. N., Van Deventer S. J. Inflammatory bowel disease is associated with increased mucosal levels of bactericidal/permeability-increasing protein. Gastroenterology. 1996 Mar;110(3):733–739. doi: 10.1053/gast.1996.v110.pm8608882. [DOI] [PubMed] [Google Scholar]
- Nicholls S., Stephens S., Braegger C. P., Walker-Smith J. A., MacDonald T. T. Cytokines in stools of children with inflammatory bowel disease or infective diarrhoea. J Clin Pathol. 1993 Aug;46(8):757–760. doi: 10.1136/jcp.46.8.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullman W. E., Elsbury S., Kobayashi M., Hapel A. J., Doe W. F. Enhanced mucosal cytokine production in inflammatory bowel disease. Gastroenterology. 1992 Feb;102(2):529–537. doi: 10.1016/0016-5085(92)90100-d. [DOI] [PubMed] [Google Scholar]
- Raab Y., Gerdin B., Ahlstedt S., Hällgren R. Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin-8 in ulcerative colitis. Gut. 1993 Sep;34(9):1203–1206. doi: 10.1136/gut.34.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989 Jan 14;298(6666):82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I., Sampson-Johannes A., Fong S., Lowe D., Min H. Y., Lin L. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J Immunol. 1992 Feb 1;148(3):808–814. [PubMed] [Google Scholar]
- Reinecker H. C., Steffen M., Witthoeft T., Pflueger I., Schreiber S., MacDermott R. P., Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993 Oct;94(1):174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saverymuttu S. H., Chadwick V. S., Hodgson H. J. Granulocyte migration in ulcerative colitis. Eur J Clin Invest. 1985 Apr;15(2):60–63. doi: 10.1111/j.1365-2362.1985.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S. H., Peters A. M., Lavender J. P., Chadwick V. S., Hodgson H. J. In vivo assessment of granulocyte migration to diseased bowel in Crohn's disease. Gut. 1985 Apr;26(4):378–383. doi: 10.1136/gut.26.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saverymuttu S. H., Peters A. M., Lavender J. P., Pepys M. B., Hodgson H. J., Chadwick V. S. Quantitative fecal indium 111-labeled leukocyte excretion in the assessment of disease in Crohn's disease. Gastroenterology. 1983 Dec;85(6):1333–1339. [PubMed] [Google Scholar]
- Schreiber S., Heinig T., Thiele H. G., Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995 May;108(5):1434–1444. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Raedler A., Conn A. R., Rombeau J. L., MacDermott R. P. Increased in vitro release of soluble interleukin 2 receptor by colonic lamina propria mononuclear cells in inflammatory bowel disease. Gut. 1992 Feb;33(2):236–241. doi: 10.1136/gut.33.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Raedler A., Stenson W. F., MacDermott R. P. The role of the mucosal immune system in inflammatory bowel disease. Gastroenterol Clin North Am. 1992 Jun;21(2):451–502. [PubMed] [Google Scholar]
- Schölmerich J., Schmidt E., Schümichen C., Billmann P., Schmidt H., Gerok W. Scintigraphic assessment of bowel involvement and disease activity in Crohn's disease using technetium 99m-hexamethyl propylene amine oxine as leukocyte label. Gastroenterology. 1988 Nov;95(5):1287–1293. doi: 10.1016/0016-5085(88)90363-0. [DOI] [PubMed] [Google Scholar]
- Stevens C., Walz G., Singaram C., Lipman M. L., Zanker B., Muggia A., Antonioli D., Peppercorn M. A., Strom T. B. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci. 1992 Jun;37(6):818–826. doi: 10.1007/BF01300378. [DOI] [PubMed] [Google Scholar]
- Vieira P., de Waal-Malefyt R., Dang M. N., Johnson K. E., Kastelein R., Fiorentino D. F., deVries J. E., Roncarolo M. G., Mosmann T. R., Moore K. W. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., Roncarolo M. G., Spits H., de Vries J. E. Interleukin-10. Curr Opin Immunol. 1992 Jun;4(3):314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- van Deventer S. J., Elson C. O., Fedorak R. N. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Crohn's Disease Study Group. Gastroenterology. 1997 Aug;113(2):383–389. doi: 10.1053/gast.1997.v113.pm9247454. [DOI] [PubMed] [Google Scholar]