Abstract
Background—Stronger prokinetic agents which specifically enhance transit in different parts of the gut are required. R093877 is a novel 5-HT4 agonist prokinetic compound which is chemically related to cisapride but believed to have greater effect on colonic activity. Aims—To evaluate the effects of R093877 on bowel function, upper and lower gut transit, visceral sensitivity, and sphincter function in healthy volunteers in a double blind, placebo controlled, crossover study. Methods—The study consisted of five consecutive one week periods: no drug treatment; active drug treatment with either 1 or 2 mg daily or placebo; washout; active drug or placebo; no treatment. Seventeen male subjects maintained a detailed diary of bowel function for the entire study. Orocaecal transit (breath hydrogen), whole gut transit (radio-opaque markers), and anorectal function were assessed at the end of each of the two treatment periods. Blood testing was performed to confirm compliance and for safety analysis. Results—One subject withdrew from the study due to side effects while on placebo. Eight subjects completed the study on 1mg and a further eight on 2 mg. Blood testing showed non-compliance in one subject on the 2 mg dose, and he was excluded from analysis of all diary and physiological data. Treatment increased the number of stools per week (placebo versus 1 mg, 7.8 versus 13.6, p=0.003; placebo versus 2 mg, 8.9 versus 11.3, p=0.15) and the percentage of loose or watery stools (24.2% versus 61.5%, p<0.04; 9.9% versus 40.0%, p<0.02). Stool frequency and consistency reverted to normal immediately after treatment. Treatment shortened orocaecal and whole gut transit in all subjects on both doses. Treatment accelerated orocaecal (76 versus 51 minutes, p=0.007; 63 versus 47 minutes, p=0.07) and whole gut (38.2 versus 27.0 hours, p=0.05; 44.8 versus 24.0 hours, p<0.04) transit, and decreased the number of retained markers ingested 36 hours previously (4.8 versus 1.8, p=0.016; 7.0 versus 4.3, p=0.033). Gut sensitivity to distension and electrical stimulation, and anal manometry, were unchanged. Transient headache occurred in seven subjects on R093877 and one subject had mild elevation of liver aminotransferases which resolved on drug cessation. Conclusions—R093877 is well tolerated by healthy subjects and has a marked and consistent effect on stool frequency and consistency, and upper gut and colonic transit. It does not affect visceral sensitivity or sphincter function. It holds promise for patients with large bowel symptoms or slow gut transit.
Keywords: idiopathic constipation; enterokinetic agents; intestinal transit; cisapride
Full Text
The Full Text of this article is available as a PDF (120.2 KB).
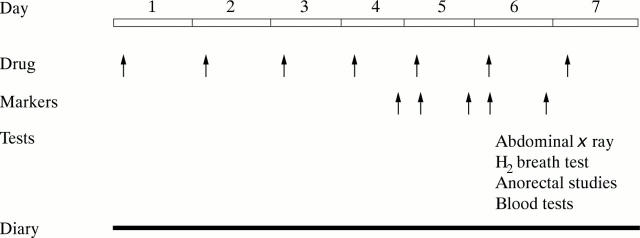
Figure 1 .
Linear representation for study protocol during each treatment week.
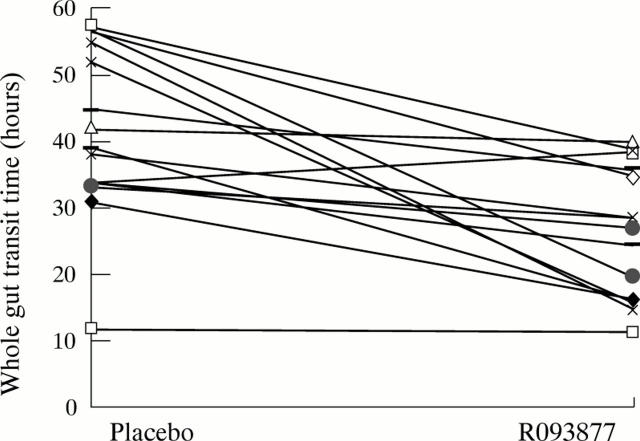
Figure 2 .
Whole gut transit time for each subject on placebo and active drug. Data shown for all 15 compliant subjects on both doses of active drug.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arhan P., Devroede G., Jehannin B., Lanza M., Faverdin C., Dornic C., Persoz B., Tétreault L., Perey B., Pellerin D. Segmental colonic transit time. Dis Colon Rectum. 1981 Nov-Dec;24(8):625–629. doi: 10.1007/BF02605761. [DOI] [PubMed] [Google Scholar]
- Barone J. A., Jessen L. M., Colaizzi J. L., Bierman R. H. Cisapride: a gastrointestinal prokinetic drug. Ann Pharmacother. 1994 Apr;28(4):488–500. doi: 10.1177/106002809402800413. [DOI] [PubMed] [Google Scholar]
- Catnach S. M., Fairclough P. D. Erythromycin and the gut. Gut. 1992 Mar;33(3):397–401. doi: 10.1136/gut.33.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussade S., Roche H., Khyari A., Couturier D., Guerre J. Mesure du temps de transit colique (TTC): description et validation d'une nouvelle technique. Gastroenterol Clin Biol. 1986 May;10(5):385–389. [PubMed] [Google Scholar]
- Hoyle C. H., Kamm M. A., Burnstock G., Lennard-Jones J. E. Enkephalins modulate inhibitory neuromuscular transmission in circular muscle of human colon via delta-opioid receptors. J Physiol. 1990 Dec;431:465–478. doi: 10.1113/jphysiol.1990.sp018340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm M. A., Lennard-Jones J. E. Rectal mucosal electrosensory testing--evidence for a rectal sensory neuropathy in idiopathic constipation. Dis Colon Rectum. 1990 May;33(5):419–423. doi: 10.1007/BF02156270. [DOI] [PubMed] [Google Scholar]
- Lux G., Katschinski M., Ludwig S., Lederer P., Ellermann A., Domschke W. The effect of cisapride and metoclopramide on human digestive and interdigestive antroduodenal motility. Scand J Gastroenterol. 1994 Dec;29(12):1105–1110. doi: 10.3109/00365529409094895. [DOI] [PubMed] [Google Scholar]
- Metcalf A. M., Phillips S. F., Zinsmeister A. R., MacCarty R. L., Beart R. W., Wolff B. G. Simplified assessment of segmental colonic transit. Gastroenterology. 1987 Jan;92(1):40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- Meulemans A. L., Schuurkes J. A. Is the action of cisapride on the guinea-pig ileum mediated via 5-HT4 receptors? Eur J Pharmacol. 1992 Feb 25;212(1):51–59. doi: 10.1016/0014-2999(92)90071-b. [DOI] [PubMed] [Google Scholar]
- O'Brien J. D., Thompson D. G., Burnham W. R., Holly J., Walker E. Action of centrally mediated autonomic stimulation on human upper gastrointestinal transit: a comparative study of two stimuli. Gut. 1987 Aug;28(8):960–969. doi: 10.1136/gut.28.8.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters T. L. Erythromycin and other macrolides as prokinetic agents. Gastroenterology. 1993 Dec;105(6):1886–1899. doi: 10.1016/0016-5085(93)91089-z. [DOI] [PubMed] [Google Scholar]
- Pilot M. A. Macrolides in roles beyond antibiotic therapy. Br J Surg. 1994 Oct;81(10):1423–1429. doi: 10.1002/bjs.1800811006. [DOI] [PubMed] [Google Scholar]
- Ramirez B., Richter J. E. Review article: promotility drugs in the treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1993 Feb;7(1):5–20. doi: 10.1111/j.1365-2036.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Reboa G., Arnulfo G., Frascio M., Di Somma C., Pitto G., Berti-Riboli E. Colon motility and colo-anal reflexes in chronic idiopathic constipation. Effects of a novel enterokinetic agent cisapride. Eur J Clin Pharmacol. 1984;26(6):745–748. doi: 10.1007/BF00541936. [DOI] [PubMed] [Google Scholar]
- Staiano A., Cucchiara S., Andreotti M. R., Minella R., Manzi G. Effect of cisapride on chronic idiopathic constipation in children. Dig Dis Sci. 1991 Jun;36(6):733–736. doi: 10.1007/BF01311229. [DOI] [PubMed] [Google Scholar]
- Tack J., Coremans G., Janssens J. A risk-benefit assessment of cisapride in the treatment of gastrointestinal disorders. Drug Saf. 1995 Jun;12(6):384–392. doi: 10.2165/00002018-199512060-00004. [DOI] [PubMed] [Google Scholar]
- Wiseman L. R., Faulds D. Cisapride. An updated review of its pharmacology and therapeutic efficacy as a prokinetic agent in gastrointestinal motility disorders. Drugs. 1994 Jan;47(1):116–152. doi: 10.2165/00003495-199447010-00008. [DOI] [PubMed] [Google Scholar]