Abstract
Background—Up to 15% of colorectal cancers are characterised by DNA microsatellite instability (MIN), shown by the presence of DNA replication errors (RERs). Aims—To identify pathological features that are discriminating for colorectal cancer (CRC) showing extensive MIN. Subjects—A prospective series of 303 patients with CRC and no family history of either familial adenomatous polyposis or hereditary non-polyposis colorectal cancer. Methods—DNA was extracted from fresh tissue samples and the presence of MIN was studied at nine loci that included TGFβRII, IGFIIR, and BAX. The 61 cases showing RERs were compared with 63 RER negative cases with respect to a comprehensive set of clinical and pathological variables. Predictive utility of the variables was tested by decision tree analysis. Results—Twenty seven patients with CRC showed extensive RERs (three loci or more) (RER+) and 34 had limited RERs only (28= one locus; 6 = two loci) (RER+/−), yielding a bimodal distribution. RER+ cancers differed from RER− and RER+/− cases. Tumour type (adenocarcinoma, mucinous carcinoma, and undifferentiated carcinoma) (p=0.001), tumour infiltrating lymphocytes (p=0.001), and anatomical site (p=0.001) were the most significant of the discriminating variables. Algorithms developed by decision tree analysis allowed cases to be assigned to RER+ versus RER− and +/− status with a global sensitivity of 81.5%, specificity of 96%, and overall accuracy of 93%. Conclusion—Pathological examination of CRC allows assignment of RER+ status; assignment is specific and relatively sensitive. Conversely RER− and RER+/− CRC are indistinguishable.
Keywords: colon; rectum; colorectal cancer; DNA replication errors; morphology; microsatellite instability
Full Text
The Full Text of this article is available as a PDF (208.0 KB).
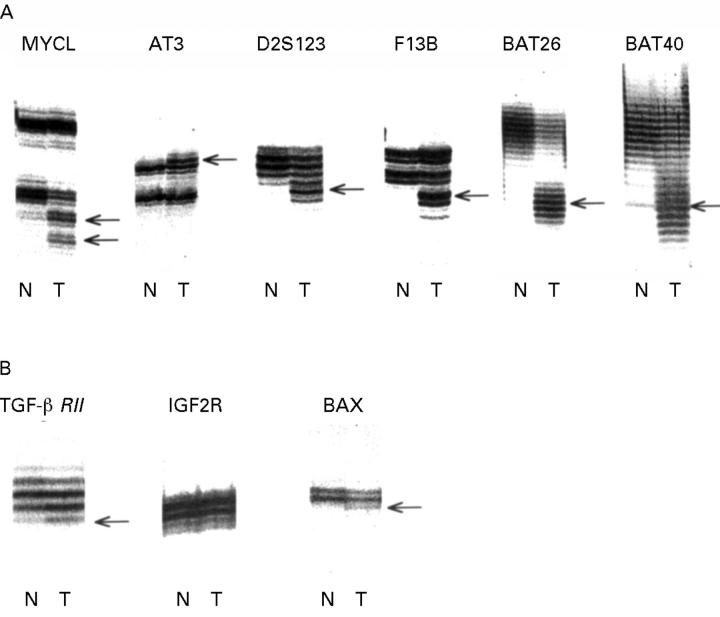
Figure 1 .
Example of cancer with extensive microsatellite instability implicating all six non-coding loci (row A) and TGFβRII and BAX in row B. The TGFβRII mutation is the most difficult to interpret, but confirmation by direct sequencing was achieved in seven cases including the one illustrated.25
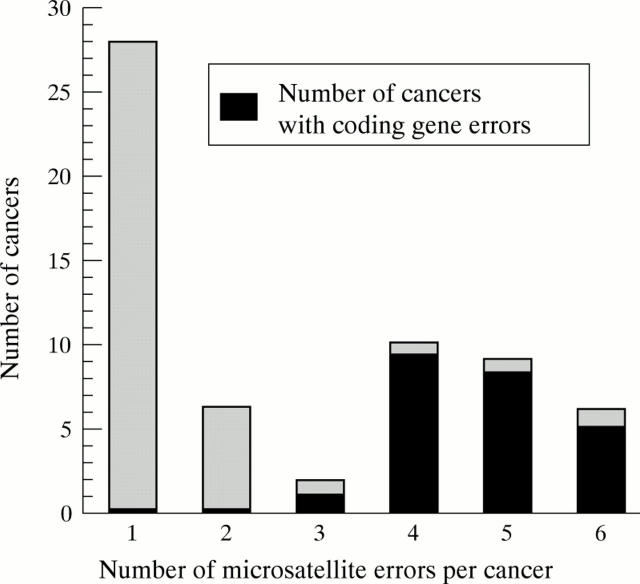
Figure 2 .
Number of microsatellite errors per cancer, showing cancers with errors in at least one of the coding genes: TGFβRII, IGFIIR, or BAX.
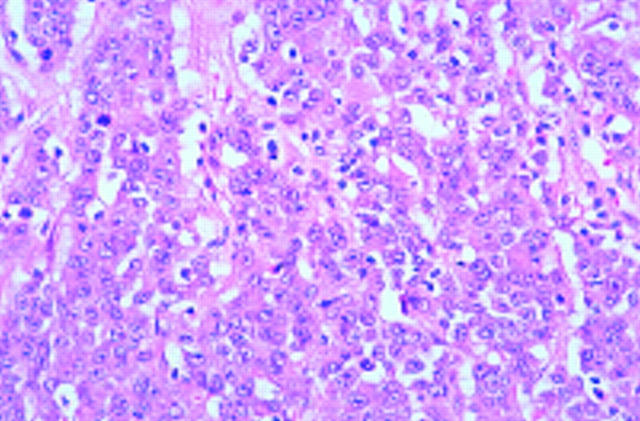
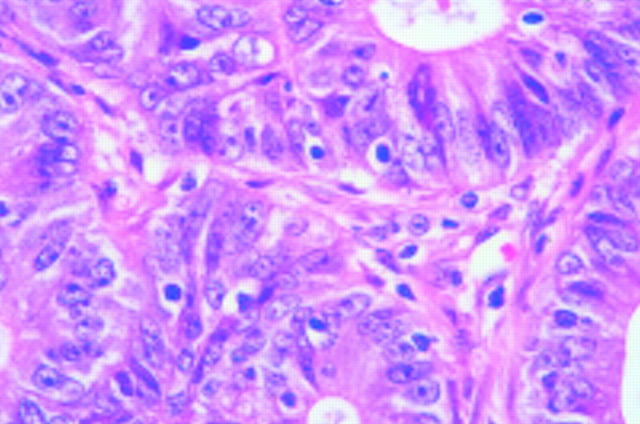
Figure 3 .
A well differentiated RER+ mucinous adenocarcinoma. Haematoxylin & eosin.
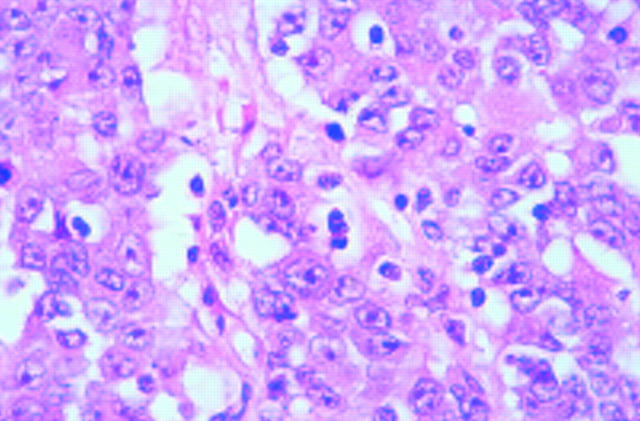
Figure 4 .
An undifferentiated RER+ carcinoma composed of solid aggregates of large cells. The tumour had a pushing margin (not shown). Foci of glandular differentiation were present (see fig 5). Haematoxylin & eosin.
Figure 5 .
High power view of fig 4 showing occasional lumina indicative of poorly differentiated adenocarcinoma. Tumour infiltrating lymphocytes are peppered throughout and many are intraepithelial. Haematoxylin & eosin.
Figure 6 .
Moderately differentiated RER+ adenocarcinoma showing intraepithelial lymphocytes with retraction artefact. Distinction from mitoses and apoptotic bodies is straightforward. Haematoxylin & eosin.
Figure 7 .
Decision tree analysis for adenocarcinomas. The ovals show the intermediate nodes and the boxes show the classifying groups and specify whether cancers are RER− or +/− versus RER+. Each box shows the sample size in the subgroup and the number that were correctly classified. Assessment of the predictive power of each variable is through the variable importance ranking and not the level shown on the tree. Relative rankings are: tumour infiltrating lymphocytes (TIL), 100; differentiation, 46; and site (RC), 13.
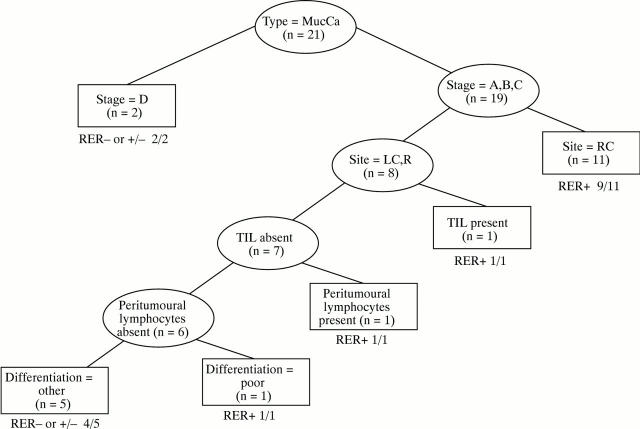
Figure 8 .
Decision tree analysis for mucinous carcinoma. Relative rankings are: stage D, 100; differentiation, 63; site (RC), 55; peritumoural lymphocytes, 45; and tumour infiltrating lymphocytes (TIL), 34.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Akiyama Y., Iwanaga R., Saitoh K., Shiba K., Ushio K., Ikeda E., Iwama T., Nomizu T., Yuasa Y. Transforming growth factor beta type II receptor gene mutations in adenomas from hereditary nonpolyposis colorectal cancer. Gastroenterology. 1997 Jan;112(1):33–39. doi: 10.1016/s0016-5085(97)70216-6. [DOI] [PubMed] [Google Scholar]
- Bland P. W., Britton D. C., Richens E. R., Pledger J. V. Peripheral, mucosal, and tumour-infiltrating components of cellular immunity in cancer of the large bowel. Gut. 1981 Sep;22(9):744–751. doi: 10.1136/gut.22.9.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocker T., Schlegel J., Kullmann F., Stumm G., Zirngibl H., Epplen J. T., Rüschoff J. Genomic instability in colorectal carcinomas: comparison of different evaluation methods and their biological significance. J Pathol. 1996 May;179(1):15–19. doi: 10.1002/(SICI)1096-9896(199605)179:1<15::AID-PATH553>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Branch P., Bicknell D. C., Rowan A., Bodmer W. F., Karran P. Immune surveillance in colorectal carcinoma. Nat Genet. 1995 Mar;9(3):231–232. doi: 10.1038/ng0395-231. [DOI] [PubMed] [Google Scholar]
- Bronner C. E., Baker S. M., Morrison P. T., Warren G., Smith L. G., Lescoe M. K., Kane M., Earabino C., Lipford J., Lindblom A. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994 Mar 17;368(6468):258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- Børresen A. L., Lothe R. A., Meling G. I., Lystad S., Morrison P., Lipford J., Kane M. F., Rognum T. O., Kolodner R. D. Somatic mutations in the hMSH2 gene in microsatellite unstable colorectal carcinomas. Hum Mol Genet. 1995 Nov;4(11):2065–2072. doi: 10.1093/hmg/4.11.2065. [DOI] [PubMed] [Google Scholar]
- Carethers J. M., Hawn M. T., Chauhan D. P., Luce M. C., Marra G., Koi M., Boland C. R. Competency in mismatch repair prohibits clonal expansion of cancer cells treated with N-methyl-N'-nitro-N-nitrosoguanidine. J Clin Invest. 1996 Jul 1;98(1):199–206. doi: 10.1172/JCI118767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawkwell L., Li D., Lewis F. A., Martin I., Dixon M. F., Quirke P. Microsatellite instability in colorectal cancer: improved assessment using fluorescent polymerase chain reaction. Gastroenterology. 1995 Aug;109(2):465–471. doi: 10.1016/0016-5085(95)90334-8. [DOI] [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Gibbs N. M. Undifferentiated carcinoma of the large intestine. Histopathology. 1977 Jan;1(1):77–84. doi: 10.1111/j.1365-2559.1977.tb01645.x. [DOI] [PubMed] [Google Scholar]
- Horii A., Han H. J., Shimada M., Yanagisawa A., Kato Y., Ohta H., Yasui W., Tahara E., Nakamura Y. Frequent replication errors at microsatellite loci in tumors of patients with multiple primary cancers. Cancer Res. 1994 Jul 1;54(13):3373–3375. [PubMed] [Google Scholar]
- Ilyas M., Tomlinson I. P., Novelli M. R., Hanby A., Bodmer W. F., Talbot I. C. Clinico-pathological features and p53 expression in left-sided sporadic colorectal cancers with and without microsatellite instability. J Pathol. 1996 Aug;179(4):370–375. doi: 10.1002/(SICI)1096-9896(199608)179:4<370::AID-PATH627>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Ajioka Y., Allen J. P., Chan Y. F., Cohen R. J., Nixon J. M., Radojkovic M., Restall A. P., Stables S. R., Zwi L. J. Assessment of invasive growth pattern and lymphocytic infiltration in colorectal cancer. Histopathology. 1996 Jun;28(6):543–548. doi: 10.1046/j.1365-2559.1996.d01-467.x. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Atkin W. S., Cuzick J., Bussey H. J., Morson B. C., Northover J. M., Todd I. P. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology. 1986 May;10(5):437–459. doi: 10.1111/j.1365-2559.1986.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Smyrk T. C., Stewart S. M., Lane M. R., Lanspa S. J., Lynch H. T. Pathology of hereditary non-polyposis colorectal cancer. Anticancer Res. 1994 Jul-Aug;14(4B):1631–1634. [PubMed] [Google Scholar]
- Kim H., Jen J., Vogelstein B., Hamilton S. R. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994 Jul;145(1):148–156. [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Kikuchi-Yanoshita R., Tanaka K., Muraoka M., Onda A., Okumura Y., Kishi N., Iwama T., Mori T., Koike M. Molecular nature of colon tumors in hereditary nonpolyposis colon cancer, familial polyposis, and sporadic colon cancer. Gastroenterology. 1996 Aug;111(2):307–317. doi: 10.1053/gast.1996.v111.pm8690195. [DOI] [PubMed] [Google Scholar]
- Leach F. S., Nicolaides N. C., Papadopoulos N., Liu B., Jen J., Parsons R., Peltomäki P., Sistonen P., Aaltonen L. A., Nyström-Lahti M. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993 Dec 17;75(6):1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- Liu B., Nicolaides N. C., Markowitz S., Willson J. K., Parsons R. E., Jen J., Papadopolous N., Peltomäki P., de la Chapelle A., Hamilton S. R. Mismatch repair gene defects in sporadic colorectal cancers with microsatellite instability. Nat Genet. 1995 Jan;9(1):48–55. doi: 10.1038/ng0195-48. [DOI] [PubMed] [Google Scholar]
- Lothe R. A., Peltomäki P., Meling G. I., Aaltonen L. A., Nyström-Lahti M., Pylkkänen L., Heimdal K., Andersen T. I., Møller P., Rognum T. O. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993 Dec 15;53(24):5849–5852. [PubMed] [Google Scholar]
- Markowitz S., Wang J., Myeroff L., Parsons R., Sun L., Lutterbaugh J., Fan R. S., Zborowska E., Kinzler K. W., Vogelstein B. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995 Jun 2;268(5215):1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- Messerini L., Vitelli F., De Vitis L. R., Mori S., Calzolari A., Palmirotta R., Calabrò A., Papi L. Microsatellite instability in sporadic mucinous colorectal carcinomas: relationship to clinico-pathological variables. J Pathol. 1997 Aug;182(4):380–384. doi: 10.1002/(SICI)1096-9896(199708)182:4<380::AID-PATH871>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Miescher S., Whiteside T. L., Carrel S., von Fliedner V. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J Immunol. 1986 Mar 1;136(5):1899–1907. [PubMed] [Google Scholar]
- Myeroff L. L., Parsons R., Kim S. J., Hedrick L., Cho K. R., Orth K., Mathis M., Kinzler K. W., Lutterbaugh J., Park K. A transforming growth factor beta receptor type II gene mutation common in colon and gastric but rare in endometrial cancers with microsatellite instability. Cancer Res. 1995 Dec 1;55(23):5545–5547. [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Wei Y. F., Ruben S. M., Carter K. C., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M., Adams M. D. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994 Mar 18;263(5153):1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- Parsons R., Myeroff L. L., Liu B., Willson J. K., Markowitz S. D., Kinzler K. W., Vogelstein B. Microsatellite instability and mutations of the transforming growth factor beta type II receptor gene in colorectal cancer. Cancer Res. 1995 Dec 1;55(23):5548–5550. [PubMed] [Google Scholar]
- Rampino N., Yamamoto H., Ionov Y., Li Y., Sawai H., Reed J. C., Perucho M. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997 Feb 14;275(5302):967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- Rüschoff J., Dietmaier W., Lüttges J., Seitz G., Bocker T., Zirngibl H., Schlegel J., Schackert H. K., Jauch K. W., Hofstaedter F. Poorly differentiated colonic adenocarcinoma, medullary type: clinical, phenotypic, and molecular characteristics. Am J Pathol. 1997 May;150(5):1815–1825. [PMC free article] [PubMed] [Google Scholar]
- Sasaki O., Atkin W. S., Jass J. R. Mucinous carcinoma of the rectum. Histopathology. 1987 Mar;11(3):259–272. doi: 10.1111/j.1365-2559.1987.tb02631.x. [DOI] [PubMed] [Google Scholar]
- Schmoor C., Ulm K., Schumacher M. Comparison of the Cox model and the regression tree procedure in analysing a randomized clinical trial. Stat Med. 1993 Dec 30;12(24):2351–2366. doi: 10.1002/sim.4780122411. [DOI] [PubMed] [Google Scholar]
- Souza R. F., Appel R., Yin J., Wang S., Smolinski K. N., Abraham J. M., Zou T. T., Shi Y. Q., Lei J., Cottrell J. Microsatellite instability in the insulin-like growth factor II receptor gene in gastrointestinal tumours. Nat Genet. 1996 Nov;14(3):255–257. doi: 10.1038/ng1196-255. [DOI] [PubMed] [Google Scholar]
- Souza R. F., Lei J., Yin J., Appel R., Zou T. T., Zhou X., Wang S., Rhyu M. G., Cymes K., Chan O. A transforming growth factor beta 1 receptor type II mutation in ulcerative colitis-associated neoplasms. Gastroenterology. 1997 Jan;112(1):40–45. doi: 10.1016/s0016-5085(97)70217-8. [DOI] [PubMed] [Google Scholar]
- Strand M., Prolla T. A., Liskay R. M., Petes T. D. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature. 1993 Sep 16;365(6443):274–276. doi: 10.1038/365274a0. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Yoo Y. K., Heo D. S., Hata K., Van Thiel D. H., Whiteside T. L. Tumor-infiltrating lymphocytes from human colon carcinomas. Functional and phenotypic characteristics after long-term culture in recombinant interleukin 2. Gastroenterology. 1990 Feb;98(2):259–268. [PubMed] [Google Scholar]