Abstract
Background—Cytokines secreted by intestinal T lymphocytes probably play a critical role in regulation of the gut associated immune responses. Aims—To quantify interferon γ (IFN-γ) and interleukin 4 (IL-4) secreting cells (SC) among human intraepithelial (IEL) and lamina propria (LPL) lymphocytes from the duodenum and right colon in non-pathological situations and in the absence of in vitro stimulation. Patients—Duodenal and right colonic biopsy specimens were obtained from patients with no inflammation of the intestinal mucosa. Methods—Intraepithelial and lamina propria cell suspensions were assayed for numbers of cells spontaneously secreting IFN-γ and IL-4 by a two site reverse enzyme linked immunospot technique (ELISPOT). Results—The relatively high proportion of duodenal lymphocytes spontaneously secreting IFN-γ (IEL 3.6%; LPL 1.9%) and IL-4 (IEL 1.3%; LPL 0.7%) contrasted with the very low numbers of spontaneously IFN-γ SC and the absence of spontaneously IL-4 SC among peripheral blood mononuclear cells. In the basal state, both IFN-γ and IL-4 were mainly produced by CD4+ cells. Within the colon, only 0.2% of IEL and LPL secreted IFN-γ in the basal state, and 0.1% secreted IL-4. Conclusions—Compared with peripheral lymphocytes substantial proportions of intestinal epithelial and lamina propria lymphocytes spontaneously secrete IFN-γ and/or IL-4. These cytokines are probably involved in the normal homoeostasis of the human intestinal mucosa. Disturbances in their secretion could play a role in the pathogenesis of gastrointestinal diseases.
Keywords: intestinal lymphocytes; ELISPOT; interferon γ; interleukin 4
Full Text
The Full Text of this article is available as a PDF (169.4 KB).
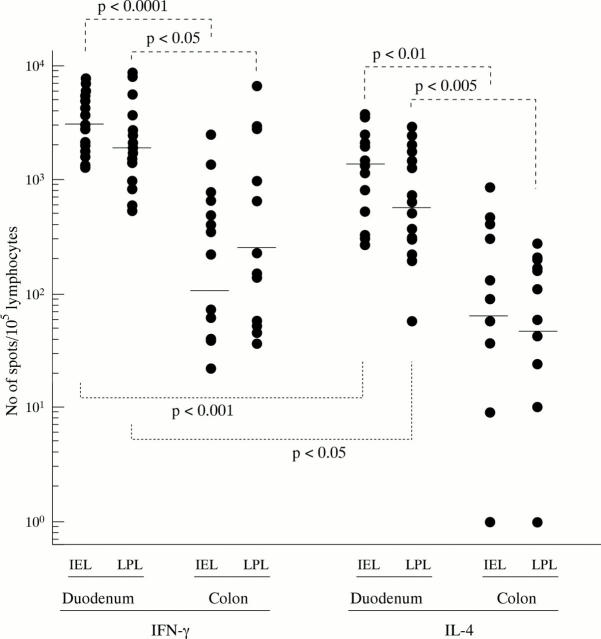
Figure 1 .
Numbers of IFN-γ and/or IL-4 secreting cells per 105 lymphocytes within IEL and LPL cell suspensions obtained from normal human intestinal duodenal and colonic mucosae. Individual results are shown and horizontal bars indicate the medians.
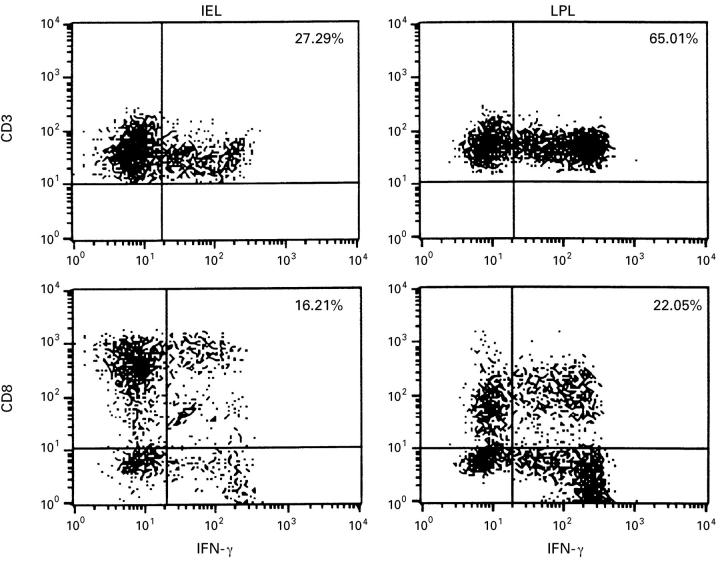
Figure 2 .
Dot plots from three colour flow cytometric analysis for anti-IFN-γ on CD3+ and on CD8+ or CD8 cells for IEL and LPL. The cells were cultured for four hours with PMA and ionophore A 23187 in the presence of brefeldin A. The fluorescence plots shown are representative of one of three different experiments and cells analysed were gated for CD3+ cells so that results are given as percentage of CD3+ cells. Control isotype was used to differentiate positive from negative immunofluorescence.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuzakouk M., Kelleher D., Feighery C., O'Farrelly C. Increased HLA-DR and decreased CD3 on human intestinal intraepithelial lymphocytes: evidence of activation? Gut. 1996 Sep;39(3):396–400. doi: 10.1136/gut.39.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett C. G., Dell'Olio D., Kontakou M., Przemioslo R. T., Rosen-Bronson S., Ciclitira P. J. Analysis of interleukin-4 and interleukin-10 and their association with the lymphocytic infiltrate in the small intestine of patients with coeliac disease. Gut. 1996 Dec;39(6):818–823. doi: 10.1136/gut.39.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S., Smith K. A. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991 Jan 1;173(1):25–36. doi: 10.1084/jem.173.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss I. J., Neurath M., Boirivant M., Klein J. S., de la Motte C., Strong S. A., Fiocchi C., Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996 Aug 1;157(3):1261–1270. [PubMed] [Google Scholar]
- Guy-Grand D., Malassis-Seris M., Briottet C., Vassalli P. Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally. Correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. J Exp Med. 1991 Jun 1;173(6):1549–1552. doi: 10.1084/jem.173.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstensen T. S., Brandtzaeg P. Activated T lymphocytes in the celiac lesion: non-proliferative activation (CD25) of CD4+ alpha/beta cells in the lamina propria but proliferation (Ki-67) of alpha/beta and gamma/delta cells in the epithelium. Eur J Immunol. 1993 Feb;23(2):505–510. doi: 10.1002/eji.1830230231. [DOI] [PubMed] [Google Scholar]
- Harvey J., Jones D. B., Wright D. H. Leucocyte common antigen expression on T cells in normal and inflamed human gut. Immunology. 1989 Sep;68(1):13–17. [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Fryklund J., Larsson H. Gamma-interferon-mediated down-regulation of electrolyte secretion by intestinal epithelial cells: a local immune mechanism? Scand J Immunol. 1989 Oct;30(4):499–503. doi: 10.1111/j.1365-3083.1989.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Jarry A., Cerf-Bensussan N., Brousse N., Selz F., Guy-Grand D. Subsets of CD3+ (T cell receptor alpha/beta or gamma/delta) and CD3- lymphocytes isolated from normal human gut epithelium display phenotypical features different from their counterparts in peripheral blood. Eur J Immunol. 1990 May;20(5):1097–1103. doi: 10.1002/eji.1830200523. [DOI] [PubMed] [Google Scholar]
- Kontakou M., Sturgess R. P., Przemioslo R. T., Limb G. A., Nelufer J. M., Ciclitira P. J. Detection of interferon gamma mRNA in the mucosa of patients with coeliac disease by in situ hybridisation. Gut. 1994 Aug;35(8):1037–1041. doi: 10.1136/gut.35.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lundqvist C., Baranov V., Hammarström S., Athlin L., Hammarström M. L. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol. 1995 Sep;7(9):1473–1487. doi: 10.1093/intimm/7.9.1473. [DOI] [PubMed] [Google Scholar]
- Lundqvist C., Melgar S., Yeung M. M., Hammarström S., Hammarström M. L. Intraepithelial lymphocytes in human gut have lytic potential and a cytokine profile that suggest T helper 1 and cytotoxic functions. J Immunol. 1996 Sep 1;157(5):1926–1934. [PubMed] [Google Scholar]
- Mosley R. L., Klein J. R. A rapid method for isolating murine intestine intraepithelial lymphocytes with high yield and purity. J Immunol Methods. 1992 Nov 25;156(1):19–26. doi: 10.1016/0022-1759(92)90006-f. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Powrie F., Menon S., Coffman R. L. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993 Sep;23(9):2223–2229. doi: 10.1002/eji.1830230926. [DOI] [PubMed] [Google Scholar]
- Quiding M., Nordström I., Kilander A., Andersson G., Hanson L. A., Holmgren J., Czerkinsky C. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-gamma production and evokes local immunological memory. J Clin Invest. 1991 Jul;88(1):143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid L. M., Gaudernack G., Markussen G., Kvale D., Brandtzaeg P., Thorsby E. Induction of various HLA class II molecules in a human colonic adenocarcinoma cell line. Scand J Immunol. 1987 Feb;25(2):175–180. doi: 10.1111/j.1365-3083.1987.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Sollid L. M., Kvale D., Brandtzaeg P., Markussen G., Thorsby E. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J Immunol. 1987 Jun 15;138(12):4303–4306. [PubMed] [Google Scholar]
- Taguchi T., McGhee J. R., Coffman R. L., Beagley K. W., Eldridge J. H., Takatsu K., Kiyono H. Analysis of Th1 and Th2 cells in murine gut-associated tissues. Frequencies of CD4+ and CD8+ T cells that secrete IFN-gamma and IL-5. J Immunol. 1990 Jul 1;145(1):68–77. [PubMed] [Google Scholar]
- Targan S. R., Deem R. L., Liu M., Wang S., Nel A. Definition of a lamina propria T cell responsive state. Enhanced cytokine responsiveness of T cells stimulated through the CD2 pathway. J Immunol. 1995 Jan 15;154(2):664–675. [PubMed] [Google Scholar]
- Testi R., Phillips J. H., Lanier L. L. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989 Mar 15;142(6):1854–1860. [PubMed] [Google Scholar]
- Trejdosiewicz L. K. Intestinal intraepithelial lymphocytes and lymphoepithelial interactions in the human gastrointestinal mucosa. Immunol Lett. 1992 Mar;32(1):13–19. doi: 10.1016/0165-2478(92)90192-q. [DOI] [PubMed] [Google Scholar]
- West G. A., Matsuura T., Levine A. D., Klein J. S., Fiocchi C. Interleukin 4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology. 1996 Jun;110(6):1683–1695. doi: 10.1053/gast.1996.v110.pm8964392. [DOI] [PubMed] [Google Scholar]
- al-Dawoud A., Nakshabendi I., Foulis A., Mowat A. M. Immunohistochemical analysis of mucosal gamma-interferon production in coeliac disease. Gut. 1992 Nov;33(11):1482–1486. doi: 10.1136/gut.33.11.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]