Abstract
Background—Endothelin-1, the most potent vasoconstrictor known, is produced in septic states and may be involved in the pathophysiology of the deteriorated splanchnic circulation seen in septic shock. Aims—To elucidate the capability of bosentan, a non-peptide mixed endothelin receptor antagonist, to attenuate splanchnic blood flow disturbances and counteract intestinal mucosal acidosis in endotoxic shock. Methods—In 16 anaesthetised pigs, central and regional haemodynamics were monitored by thermodilution and ultrasonic flow probes, respectively. A tonometer in the ileum was used for measurement of mucosal pH. Onset of endotoxin challenge was followed by bosentan administration (to eight pigs) two hours later. Results—Endotoxin infusion reduced cardiac index and systemic oxygen delivery; bosentan restored these parameters. The reduced mean arterial blood pressure and renal blood flow remained unaffected by bosentan. The profound reduction in gut oxygen delivery in response to endotoxin was completely abolished by bosentan. Bosentan significantly improved the notably deteriorated intestinal mucosal pH and mucosal-arterial PCO2 gap. The mucosal-portal vein PCO2 gap, used to monitor the mucosa in relation to the gut as a whole (including the spleen and pancreas), was also greatly increased by endotoxaemia and significantly reversed by bosentan. Conclusion—Bosentan completely restored the profound endotoxin induced reductions in systemic and gut oxygen delivery with a concomitant reversal of intestinal mucosal acidosis. Results suggest that endothelin is involved in the pronounced perfusion disturbances seen in the gut in endotoxic shock. Bosentan may prove useful in reducing gut ischaemia in septic shock.
Keywords: splanchnic circulation; septic shock; tonometry; pHi; PCO2 gap; endothelin-1
Full Text
The Full Text of this article is available as a PDF (137.7 KB).
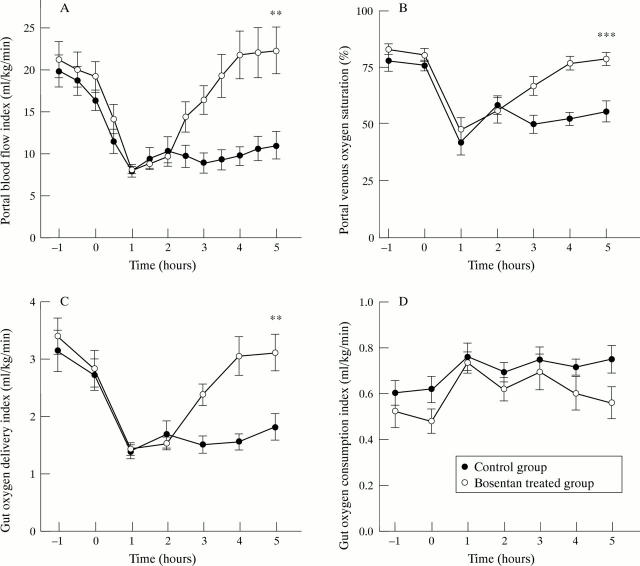
Figure 1 .
Portal blood flow index (A), portal venous oxygen saturation (B), gut oxygen delivery index (C), and gut oxygen consumption index (D). Control group, n=8; bosentan treated group, n=8. Significant differences between groups at T0h, T2h, and T5h are symbolised by **p<0.01 and ***p<0.001.
Figure 2 .
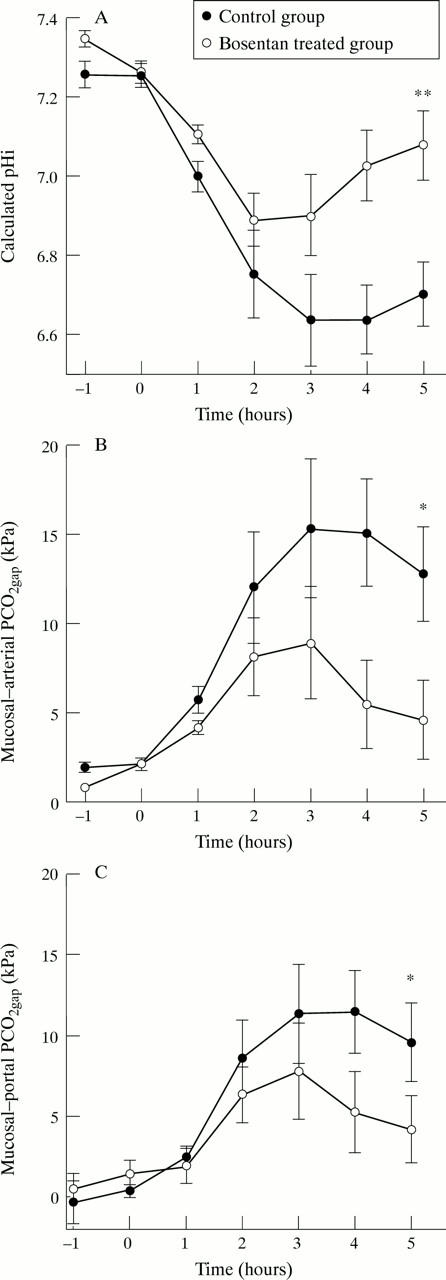
Calculated pHi (A), mucosal-arterial PCO2 gap (B), and mucosal-portal PCO2 gap (C). Control group, n=8; bosentan treated group, n=8. Significant differences between groups at T0h, T2h, and T5h are symbolised by *p<0.05 and **p<0.01.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonsson J. B., Haglund U. H. Gut intramucosal pH and intraluminal PO2 in a porcine model of peritonitis or haemorrhage. Gut. 1995 Dec;37(6):791–797. doi: 10.1136/gut.37.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranow J. S., Fink M. P. Determinants of intestinal barrier failure in critical illness. Br J Anaesth. 1996 Jul;77(1):71–81. doi: 10.1093/bja/77.1.71. [DOI] [PubMed] [Google Scholar]
- Arvidsson D., Rasmussen I., Almqvist P., Niklasson F., Haglund U. Splanchnic oxygen consumption in septic and hemorrhagic shock. Surgery. 1991 Feb;109(2):190–197. [PubMed] [Google Scholar]
- Ayuse T., Brienza N., Revelly J. P., O'Donnell C. P., Boitnott J. K., Robotham J. L. Alternations in liver hemodynamics in an intact porcine model of endotoxin shock. Am J Physiol. 1995 Mar;268(3 Pt 2):H1106–H1114. doi: 10.1152/ajpheart.1995.268.3.H1106. [DOI] [PubMed] [Google Scholar]
- Baron P., Traber L. D., Traber D. L., Nguyen T., Hollyoak M., Heggers J. P., Herndon D. N. Gut failure and translocation following burn and sepsis. J Surg Res. 1994 Jul;57(1):197–204. doi: 10.1006/jsre.1994.1131. [DOI] [PubMed] [Google Scholar]
- Benjamin E., Polokoff E., Oropello J. M., Leibowitz A. B., Iberti T. J. Sodium bicarbonate administration affects the diagnostic accuracy of gastrointestinal tonometry in acute mesenteric ischemia. Crit Care Med. 1992 Aug;20(8):1181–1183. doi: 10.1097/00003246-199208000-00019. [DOI] [PubMed] [Google Scholar]
- Biffl W. L., Moore E. E. Splanchnic ischaemia/reperfusion and multiple organ failure. Br J Anaesth. 1996 Jul;77(1):59–70. doi: 10.1093/bja/77.1.59. [DOI] [PubMed] [Google Scholar]
- Clozel M., Breu V., Gray G. A., Kalina B., Löffler B. M., Burri K., Cassal J. M., Hirth G., Müller M., Neidhart W. Pharmacological characterization of bosentan, a new potent orally active nonpeptide endothelin receptor antagonist. J Pharmacol Exp Ther. 1994 Jul;270(1):228–235. [PubMed] [Google Scholar]
- Cocks T. M., Faulkner N. L., Sudhir K., Angus J. Reactivity of endothelin-1 on human and canine large veins compared with large arteries in vitro. Eur J Pharmacol. 1989 Nov 14;171(1):17–24. doi: 10.1016/0014-2999(89)90425-1. [DOI] [PubMed] [Google Scholar]
- Dahn M. S., Lange P., Lobdell K., Hans B., Jacobs L. A., Mitchell R. A. Splanchnic and total body oxygen consumption differences in septic and injured patients. Surgery. 1987 Jan;101(1):69–80. [PubMed] [Google Scholar]
- Deitch E. A., Berg R., Specian R. Endotoxin promotes the translocation of bacteria from the gut. Arch Surg. 1987 Feb;122(2):185–190. doi: 10.1001/archsurg.1987.01400140067008. [DOI] [PubMed] [Google Scholar]
- Dupuis J., Goresky C. A., Fournier A. Pulmonary clearance of circulating endothelin-1 in dogs in vivo: exclusive role of ETB receptors. J Appl Physiol (1985) 1996 Oct;81(4):1510–1515. doi: 10.1152/jappl.1996.81.4.1510. [DOI] [PubMed] [Google Scholar]
- Fink M. P. Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit Care Med. 1991 May;19(5):627–641. doi: 10.1097/00003246-199105000-00009. [DOI] [PubMed] [Google Scholar]
- Fink M. P., Kaups K. L., Wang H. L., Rothschild H. R. Maintenance of superior mesenteric arterial perfusion prevents increased intestinal mucosal permeability in endotoxic pigs. Surgery. 1991 Aug;110(2):154–161. [PubMed] [Google Scholar]
- Gardiner S. M., Kemp P. A., March J. E., Bennett T. Effects of bosentan (Ro 47-0203), an ETA-, ETB-receptor antagonist, on regional haemodynamic responses to endothelins in conscious rats. Br J Pharmacol. 1994 Jul;112(3):823–830. doi: 10.1111/j.1476-5381.1994.tb13153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld A. B., Kolkman J. J. Splanchnic tonometry: a review of physiology, methodology, and clinical applications. J Crit Care. 1994 Sep;9(3):198–210. doi: 10.1016/0883-9441(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Gross S. S., Wolin M. S. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- Gutierrez G., Palizas F., Doglio G., Wainsztein N., Gallesio A., Pacin J., Dubin A., Schiavi E., Jorge M., Pusajo J. Gastric intramucosal pH as a therapeutic index of tissue oxygenation in critically ill patients. Lancet. 1992 Jan 25;339(8787):195–199. doi: 10.1016/0140-6736(92)90002-k. [DOI] [PubMed] [Google Scholar]
- Gårdebäck M., Settergren G., Ohquist G., Tirén C. Effect of dopexamine on calculated low gastric intramucosal pH following valve replacement. Acta Anaesthesiol Scand. 1995 Jul;39(5):599–604. doi: 10.1111/j.1399-6576.1995.tb04134.x. [DOI] [PubMed] [Google Scholar]
- Helset E., Ytrehus K., Tveita T., Kjaeve J., Jørgensen L. Endothelin-1 causes accumulation of leukocytes in the pulmonary circulation. Circ Shock. 1994 Dec;44(4):201–209. [PubMed] [Google Scholar]
- Hemsén A. Biochemical and functional characterization of endothelin peptides with special reference to vascular effects. Acta Physiol Scand Suppl. 1991;602:1–61. [PubMed] [Google Scholar]
- Hemsén A., Modin A., Wanecek M., Malmström R. E., Weitzberg E. Effects of Ro 47-0203 and PD155080 on the plasma kinetics, receptor binding and vascular effects of endothelin in the pig. Eur J Pharmacol. 1996 Dec 30;318(2-3):369–376. doi: 10.1016/s0014-2999(96)00807-2. [DOI] [PubMed] [Google Scholar]
- Humer M. F., Phang P. T., Friesen B. P., Allard M. F., Goddard C. M., Walley K. R. Heterogeneity of gut capillary transit times and impaired gut oxygen extraction in endotoxemic pigs. J Appl Physiol (1985) 1996 Aug;81(2):895–904. doi: 10.1152/jappl.1996.81.2.895. [DOI] [PubMed] [Google Scholar]
- Kiowski W., Sütsch G., Hunziker P., Müller P., Kim J., Oechslin E., Schmitt R., Jones R., Bertel O. Evidence for endothelin-1-mediated vasoconstriction in severe chronic heart failure. Lancet. 1995 Sep 16;346(8977):732–736. doi: 10.1016/s0140-6736(95)91504-4. [DOI] [PubMed] [Google Scholar]
- Lundblad R., Giercksky K. E. Endothelin concentrations in experimental sepsis: profiles of big endothelin and endothelin 1-21 in lethal peritonitis in rats. Eur J Surg. 1995 Jan;161(1):9–16. [PubMed] [Google Scholar]
- Löffler B. M., Breu V., Clozel M. Effect of different endothelin receptor antagonists and of the novel non-peptide antagonist Ro 46-2005 on endothelin levels in rat plasma. FEBS Lett. 1993 Oct 25;333(1-2):108–110. doi: 10.1016/0014-5793(93)80384-7. [DOI] [PubMed] [Google Scholar]
- Marik P. E., Mohedin M. The contrasting effects of dopamine and norepinephrine on systemic and splanchnic oxygen utilization in hyperdynamic sepsis. JAMA. 1994 Nov 2;272(17):1354–1357. [PubMed] [Google Scholar]
- Maynard N., Bihari D., Beale R., Smithies M., Baldock G., Mason R., McColl I. Assessment of splanchnic oxygenation by gastric tonometry in patients with acute circulatory failure. JAMA. 1993 Sep 8;270(10):1203–1210. [PubMed] [Google Scholar]
- Montgomery A., Almqvist P., Arvidsson D., Lindgren S., Haglund U. Early detection of gastrointestinal mucosal ischemia in porcine E. coli sepsis. Acta Chir Scand. 1990 Sep;156(9):613–620. [PubMed] [Google Scholar]
- Morise Z., Ueda M., Aiura K., Endo M., Kitajima M. Pathophysiologic role of endothelin-1 in renal function in rats with endotoxin shock. Surgery. 1994 Feb;115(2):199–204. [PubMed] [Google Scholar]
- Myhre U., Pettersen J. T., Risøe C., Giercksky K. E. Endothelin-1 and endotoxemia. J Cardiovasc Pharmacol. 1993;22 (Suppl 8):S291–S294. doi: 10.1097/00005344-199322008-00076. [DOI] [PubMed] [Google Scholar]
- Mythen M. G., Webb A. R. Intra-operative gut mucosal hypoperfusion is associated with increased post-operative complications and cost. Intensive Care Med. 1994;20(2):99–104. doi: 10.1007/BF01707662. [DOI] [PubMed] [Google Scholar]
- Oldner A., Goiny M., Ungerstedt U., Sollevi A. Splanchnic homeostasis during endotoxin challenge in the pig as assessed by microdialysis and tonometry. Shock. 1996 Sep;6(3):188–193. [PubMed] [Google Scholar]
- Parviainen I., Ruokonen E., Takala J. Dobutamine-induced dissociation between changes in splanchnic blood flow and gastric intramucosal pH after cardiac surgery. Br J Anaesth. 1995 Mar;74(3):277–282. doi: 10.1093/bja/74.3.277. [DOI] [PubMed] [Google Scholar]
- Pittet J. F., Morel D. R., Hemsen A., Gunning K., Lacroix J. S., Suter P. M., Lundberg J. M. Elevated plasma endothelin-1 concentrations are associated with the severity of illness in patients with sepsis. Ann Surg. 1991 Mar;213(3):261–264. doi: 10.1097/00000658-199103000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen I., Haglund U. Early gut ischemia in experimental fecal peritonitis. Circ Shock. 1992 Sep;38(1):22–28. [PubMed] [Google Scholar]
- Reeder L. B., Ferguson M. K. Endothelin-1 synthesis and receptor-mediated activity in porcine lymph vessels. J Surg Res. 1996 Jun;63(1):215–219. doi: 10.1006/jsre.1996.0250. [DOI] [PubMed] [Google Scholar]
- Revelly J. P., Ayuse T., Brienza N., Fessler H. E., Robotham J. L. Endotoxic shock alters distribution of blood flow within the intestinal wall. Crit Care Med. 1996 Aug;24(8):1345–1351. doi: 10.1097/00003246-199608000-00013. [DOI] [PubMed] [Google Scholar]
- Revelly J. P., Ayuse T., Brienza N., Robotham J. L. Dysregulation of the veno-arterial response in the superior mesenteric artery during endotoxic shock. Crit Care Med. 1995 Sep;23(9):1519–1527. doi: 10.1097/00003246-199509000-00012. [DOI] [PubMed] [Google Scholar]
- Ruokonen E., Takala J., Kari A., Saxén H., Mertsola J., Hansen E. J. Regional blood flow and oxygen transport in septic shock. Crit Care Med. 1993 Sep;21(9):1296–1303. doi: 10.1097/00003246-199309000-00011. [DOI] [PubMed] [Google Scholar]
- Schlichting E., Grotmol T., Kähler H., Naess O., Steinbakk M., Lyberg T. Alterations in mucosal morphology and permeability, but no bacterial or endotoxin translocation takes place after intestinal ischemia and early reperfusion in pigs. Shock. 1995 Feb;3(2):116–124. [PubMed] [Google Scholar]
- Takakuwa T., Endo S., Nakae H., Kikichi M., Suzuki T., Inada K., Yoshida M. Plasma levels of TNF-alpha, endothelin-1 and thrombomodulin in patients with sepsis. Res Commun Chem Pathol Pharmacol. 1994 Jun;84(3):261–269. [PubMed] [Google Scholar]
- Thiemermann C. The role of the L-arginine: nitric oxide pathway in circulatory shock. Adv Pharmacol. 1994;28:45–79. doi: 10.1016/s1054-3589(08)60493-7. [DOI] [PubMed] [Google Scholar]
- Uusaro A., Ruokonen E., Takala J. Gastric mucosal pH does not reflect changes in splanchnic blood flow after cardiac surgery. Br J Anaesth. 1995 Feb;74(2):149–154. doi: 10.1093/bja/74.2.149. [DOI] [PubMed] [Google Scholar]
- VanderMeer T. J., Wang H., Fink M. P. Endotoxemia causes ileal mucosal acidosis in the absence of mucosal hypoxia in a normodynamic porcine model of septic shock. Crit Care Med. 1995 Jul;23(7):1217–1226. doi: 10.1097/00003246-199507000-00011. [DOI] [PubMed] [Google Scholar]
- Wanecek M., Oldner A., Rudehill A., Sollevi A., Alving K., Weitzberg E. Cardiopulmonary dysfunction during porcine endotoxin shock is effectively counteracted by the endothelin receptor antagonist bosentan. Shock. 1997 May;7(5):364–370. doi: 10.1097/00024382-199705000-00009. [DOI] [PubMed] [Google Scholar]
- Weitzberg E., Ahlborg G., Lundberg J. M. Long-lasting vasoconstriction and efficient regional extraction of endothelin-1 in human splanchnic and renal tissues. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1298–1303. doi: 10.1016/s0006-291x(05)81336-1. [DOI] [PubMed] [Google Scholar]
- Weitzberg E., Hemsén A., Rudehill A., Modin A., Wanecek M., Lundberg J. M. Bosentan-improved cardiopulmonary vascular performance and increased plasma levels of endothelin-1 in porcine endotoxin shock. Br J Pharmacol. 1996 Jun;118(3):617–626. doi: 10.1111/j.1476-5381.1996.tb15446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzberg E., Lundberg J. M., Rudehill A. Elevated plasma levels of endothelin in patients with sepsis syndrome. Circ Shock. 1991 Apr;33(4):222–227. [PubMed] [Google Scholar]
- Wilson M. A., Steeb G. D., Garrison R. N. Endothelins mediate intestinal hypoperfusion during bacteremia. J Surg Res. 1993 Aug;55(2):168–175. doi: 10.1006/jsre.1993.1125. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Thomas R., D'Orleans-Juste P., Antunes E., Walder C., Warner T. D., Vane J. R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]