Abstract
Background—Reactive oxygen species and related oxidative damage have been implicated in the initiation of acute pancreatitis. Changes in these parameters during disease progression merit further investigation. Aims—To evaluate changes and the clinical relevance of superoxide radicals, endogenous antioxidants, and lipid peroxidation during the course of acute pancreatitis. Patients and methods—Superoxide radicals (measured as lucigenin amplified chemiluminescence), ascorbic acid, dehydroascorbic acid, α tocopherol, and lipid peroxidation (measured as thiobarbiturate reactive substances) were analysed in blood samples from 56 healthy subjects, 30 patients with mild acute pancreatitis, and 23 patients with severe acute pancreatitis. The association with grades of disease severity was analysed. Measurements were repeated one and two weeks after onset of pancreatitis. Results—In the blood from patients with acute pancreatitis, there were increased levels of the superoxide radical as well as lipid peroxides. There was notable depletion of ascorbic acid and an increased fraction of dehydroascorbic acid. Changes in α tocopherol were not great except in one case with poor prognosis. Differences between severe and mild acute pancreatitis were significant (p<0.01). Variable but significant correlations with disease severity scores were found for most of these markers. The normalisation of these indexes postdated clinical recovery one or two weeks after onset of disease. Conclusions—Heightened oxidative stress appears early in the course of acute pancreatitis and lasts longer than the clinical manifestations. The dependence of disease severity on the imbalance between oxidants and natural defences suggests that oxidative stress may have a pivotal role in the progression of pancreatitis and may provide a target for treatment.
Keywords: acute pancreatitis; free radicals; superoxides; antioxidants; lipid peroxidation
Full Text
The Full Text of this article is available as a PDF (146.5 KB).
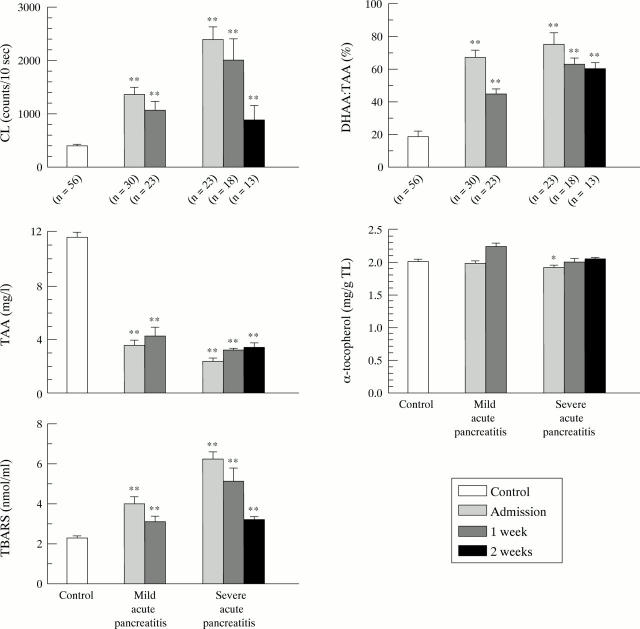
Figure 1 .
Follow up measurements of lucigenin amplified chemiluminescence (CL), total ascorbic acid (TAA), dehydroascorbic acid (DHAA), α tocopherol, and thiobarbituric acid reactive substances (TBARS) in patients with mild or severe acute pancreatitis. *p<0.05, **p<0.01 versus controls.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björk J., Arfors K. E. Oxygen free radicals and leukotriene B4 induced increase in vascular leakage is mediated by polymorphonuclear leukocytes. Agents Actions Suppl. 1982;11:63–72. [PubMed] [Google Scholar]
- Bradley E. L., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993 May;128(5):586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- Braganza J. M., Holmes A. M., Morton A. R., Stalley L., Ku R., Kisher R. Acetylcysteine to treat complications of pancreatitis. Lancet. 1986 Apr 19;1(8486):914–915. doi: 10.1016/s0140-6736(86)91017-2. [DOI] [PubMed] [Google Scholar]
- Braganza J. M., Scott P., Bilton D., Schofield D., Chaloner C., Shiel N., Hunt L. P., Bottiglieri T. Evidence for early oxidative stress in acute pancreatitis. Clues for correction. Int J Pancreatol. 1995 Feb;17(1):69–81. doi: 10.1007/BF02788361. [DOI] [PubMed] [Google Scholar]
- Büchler M., Malfertheiner P., Uhl W., Schölmerich J., Stöckmann F., Adler G., Gaus W., Rolle K., Beger H. G. Gabexate mesilate in human acute pancreatitis. German Pancreatitis Study Group. Gastroenterology. 1993 Apr;104(4):1165–1170. doi: 10.1016/0016-5085(93)90288-n. [DOI] [PubMed] [Google Scholar]
- Catignani G. L., Bieri J. G. Simultaneous determination of retinol and alpha-tocopherol in serum or plasma by liquid chromatography. Clin Chem. 1983 Apr;29(4):708–712. [PubMed] [Google Scholar]
- Chardavoyne R., Asher A., Bank S., Stein T. A., Wise L. Role of reactive oxygen metabolites in early cardiopulmonary changes of acute hemorrhagic pancreatitis. Dig Dis Sci. 1989 Oct;34(10):1581–1584. doi: 10.1007/BF01537114. [DOI] [PubMed] [Google Scholar]
- De Waele B., Vierendeels T., Willems G. Vitamin status in patients with acute pancreatitis. Clin Nutr. 1992 Apr;11(2):83–86. doi: 10.1016/0261-5614(92)90015-i. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Glazer A. N. Phycoerythrin fluorescence-based assay for peroxy radicals: a screen for biologically relevant protective agents. Anal Biochem. 1989 Mar;177(2):300–306. doi: 10.1016/0003-2697(89)90056-0. [DOI] [PubMed] [Google Scholar]
- Dormandy T. L., Wickens D. G. The experimental and clinical pathology of diene conjugation. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):353–364. doi: 10.1016/0009-3084(87)90072-7. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Schaur R. J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Esterline R. L., Trush M. A. Lucigenin chemiluminescence and its relationship to mitochondrial respiration in phagocytic cells. Biochem Biophys Res Commun. 1989 Mar 15;159(2):584–591. doi: 10.1016/0006-291x(89)90034-x. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Faulkner K., Fridovich I. Luminol and lucigenin as detectors for O2.-. Free Radic Biol Med. 1993 Oct;15(4):447–451. doi: 10.1016/0891-5849(93)90044-u. [DOI] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode H. F., Cowley H. C., Walker B. E., Howdle P. D., Webster N. R. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit Care Med. 1995 Apr;23(4):646–651. doi: 10.1097/00003246-199504000-00011. [DOI] [PubMed] [Google Scholar]
- Gough D. B., Boyle B., Joyce W. P., Delaney C. P., McGeeney K. F., Gorey T. F., Fitzpatrick J. M. Free radical inhibition and serial chemiluminescence in evolving experimental pancreatitis. Br J Surg. 1990 Nov;77(11):1256–1259. doi: 10.1002/bjs.1800771119. [DOI] [PubMed] [Google Scholar]
- Guice K. S., Oldham K. T., Caty M. G., Johnson K. J., Ward P. A. Neutrophil-dependent, oxygen-radical mediated lung injury associated with acute pancreatitis. Ann Surg. 1989 Dec;210(6):740–747. doi: 10.1097/00000658-198912000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyan P. M., Uden S., Braganza J. M. Heightened free radical activity in pancreatitis. Free Radic Biol Med. 1990;8(4):347–354. doi: 10.1016/0891-5849(90)90100-w. [DOI] [PubMed] [Google Scholar]
- Horwitt M. K., Harvey C. C., Dahm C. H., Jr, Searcy M. T. Relationship between tocopherol and serum lipid levels for determination of nutritional adequacy. Ann N Y Acad Sci. 1972 Dec 18;203:223–236. doi: 10.1111/j.1749-6632.1972.tb27878.x. [DOI] [PubMed] [Google Scholar]
- Lu F. J., Lin J. T., Wang H. P., Huang W. C. A simple, sensitive, non-stimulated photon counting system for detection of superoxide anion in whole blood. Experientia. 1996 Feb 15;52(2):141–144. doi: 10.1007/BF01923359. [DOI] [PubMed] [Google Scholar]
- Malech H. L., Gallin J. I. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987 Sep 10;317(11):687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- Margolis S. A., Paule R. C., Ziegler R. G. Ascorbic and dehydroascorbic acids measured in plasma preserved with dithiothreitol or metaphosphoric acid. Clin Chem. 1990 Oct;36(10):1750–1755. [PubMed] [Google Scholar]
- Nonaka A., Manabe T., Tamura K., Asano N., Imanishi K., Tobe T. Changes of xanthine oxidase, lipid peroxide and superoxide dismutase in mouse acute pancreatitis. Digestion. 1989;43(1-2):41–46. doi: 10.1159/000199859. [DOI] [PubMed] [Google Scholar]
- Nonaka A., Manabe T., Tamura K., Asano N., Imanishi K., Yamaki K., Tobe T. [Organ specific ESR features in mouse main organs and ESR application to the model of pancreatic disorders]. Nihon Geka Gakkai Zasshi. 1990 Feb;91(2):169–173. [PubMed] [Google Scholar]
- Nonaka A., Manabe T., Tobe T. Effect of a new synthetic ascorbic acid derivative as a free radical scavenger on the development of acute pancreatitis in mice. Gut. 1991 May;32(5):528–532. doi: 10.1136/gut.32.5.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose P., Fraine E., Hunt L. P., Acheson D. W., Braganza J. M. Dietary antioxidants and chronic pancreatitis. Hum Nutr Clin Nutr. 1986 Mar;40(2):151–164. [PubMed] [Google Scholar]
- Sanfey H., Bulkley G. B., Cameron J. L. The pathogenesis of acute pancreatitis. The source and role of oxygen-derived free radicals in three different experimental models. Ann Surg. 1985 May;201(5):633–639. doi: 10.1097/00000658-198505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M. H., Birk D., Beger H. G. Oxidative stress in acute and chronic pancreatitis. Am J Clin Nutr. 1995 Dec;62(6 Suppl):1306S–1314S. doi: 10.1093/ajcn/62.6.1306S. [DOI] [PubMed] [Google Scholar]
- Schoenberg M. H., Büchler M., Gaspar M., Stinner A., Younes M., Melzner I., Bültmann B., Beger H. G. Oxygen free radicals in acute pancreatitis of the rat. Gut. 1990 Oct;31(10):1138–1143. doi: 10.1136/gut.31.10.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M. H., Büchler M., Pietrzyk C., Uhl W., Birk D., Eisele S., Marzinzig M., Beger H. G. Lipid peroxidation and glutathione metabolism in chronic pancreatitis. Pancreas. 1995 Jan;10(1):36–43. doi: 10.1097/00006676-199501000-00005. [DOI] [PubMed] [Google Scholar]
- Scott P., Bruce C., Schofield D., Shiel N., Braganza J. M., McCloy R. F. Vitamin C status in patients with acute pancreatitis. Br J Surg. 1993 Jun;80(6):750–754. doi: 10.1002/bjs.1800800632. [DOI] [PubMed] [Google Scholar]
- Sun J. S., Hang Y. S., Huang I. H., Lu F. J. A simple chemiluminescence assay for detecting oxidative stress in ischemic limb injury. Free Radic Biol Med. 1996;20(1):107–112. doi: 10.1016/0891-5849(95)02011-x. [DOI] [PubMed] [Google Scholar]
- Uden S., Bilton D., Nathan L., Hunt L. P., Main C., Braganza J. M. Antioxidant therapy for recurrent pancreatitis: placebo-controlled trial. Aliment Pharmacol Ther. 1990 Aug;4(4):357–371. doi: 10.1111/j.1365-2036.1990.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Wilson C., Heath D. I., Imrie C. W. Prediction of outcome in acute pancreatitis: a comparative study of APACHE II, clinical assessment and multiple factor scoring systems. Br J Surg. 1990 Nov;77(11):1260–1264. doi: 10.1002/bjs.1800771120. [DOI] [PubMed] [Google Scholar]
- Wisner J., Green D., Ferrell L., Renner I. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut. 1988 Nov;29(11):1516–1523. doi: 10.1136/gut.29.11.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]