Abstract
A greater understanding of the natural history of acute pancreatitis combined with greatly improved radiological imaging has led to improvement in the hospital mortality from acute pancreatitis, from around 25-30% to 6-10% in the past 30 years. Moreover, it is now recognised that the first phase of severe acute phase pancreatitis is a systemic inflammatory response syndrome (SIRS), during which multiple organ failure and death often supervene. Survival into the second phase may be accompanied by local complications, such as infected pancreatic necrosis, which may be prevented by prophylactic antibiotics and treated by judicious surgery. Intensive care unit costs can be substantial, but might be justified because of the excellent quality of life of survivors. Reduction in multiple organ failure by agents such as lexipafant, an antagonist of platelet activating factor (PAF) (which plays a critical role in generating the SIRS), may contribute to intensive care unit cost containment, as well as reducing the incidence of local complications and deaths from acute pancreatitis. A further improvement in the human and financial costs also requires the centralisation of the management of patients with severe acute pancreatitis, to single hospital units whose concentrated expertise equips them to intervene most effectively in what is still recognised as a highly complex disease.
Full Text
The Full Text of this article is available as a PDF (139.3 KB).
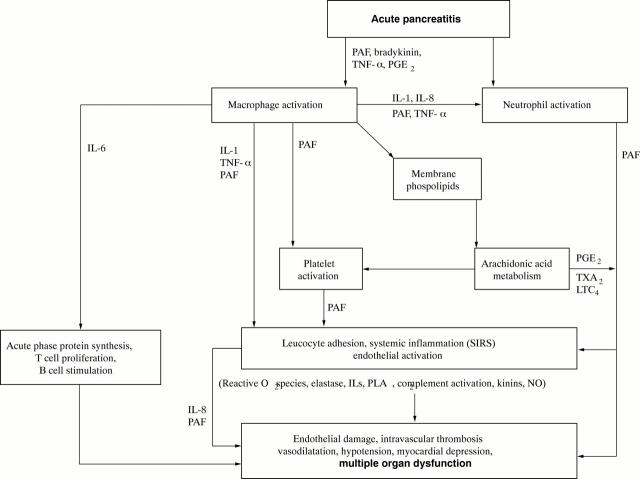
Figure 1 .
Schematic representation of the important pathways in acute pancreatitis leading to systemic inflammatory response syndrome (SIRS) and multiple organ failure. PAF, platelet activating factor; IL, interleukin, TNF-α, tumour necrosis factor α; PGE2, prostaglandin E2; TXA2, thromboxane A2; LT4, leukotriene series 4; IIs PLA2, type II (non-pancreatic) secretory phospholipase A2; NO, nitric oxide.
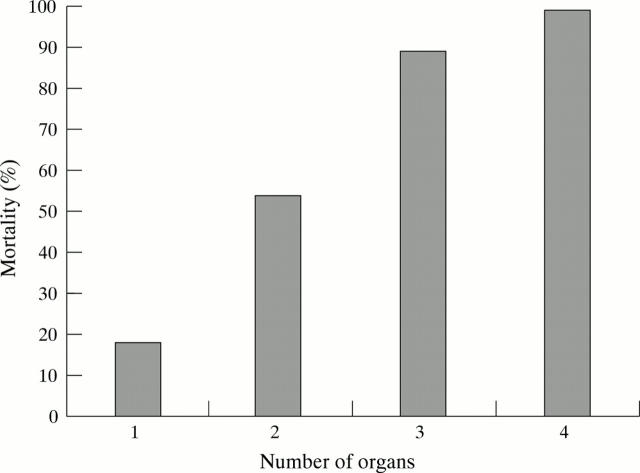
Figure 2 .
Rising mortality in acute pancreatitis with increasing number of organs affected (adapted from Heath et al26).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Shaw S. Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet. 1994 Apr 2;343(8901):831–836. doi: 10.1016/s0140-6736(94)92029-x. [DOI] [PubMed] [Google Scholar]
- Anderson B. O., Bensard D. D., Harken A. H. The role of platelet activating factor and its antagonists in shock, sepsis and multiple organ failure. Surg Gynecol Obstet. 1991 May;172(5):415–424. [PubMed] [Google Scholar]
- Balthazar E. J., Robinson D. L., Megibow A. J., Ranson J. H. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990 Feb;174(2):331–336. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- Beal A. L., Cerra F. B. Multiple organ failure syndrome in the 1990s. Systemic inflammatory response and organ dysfunction. JAMA. 1994 Jan 19;271(3):226–233. [PubMed] [Google Scholar]
- Beger H. G., Bittner R., Block S., Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986 Aug;91(2):433–438. doi: 10.1016/0016-5085(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Beger H. G., Büchler M., Bittner R., Block S., Nevalainen T., Roscher R. Necrosectomy and postoperative local lavage in necrotizing pancreatitis. Br J Surg. 1988 Mar;75(3):207–212. doi: 10.1002/bjs.1800750306. [DOI] [PubMed] [Google Scholar]
- Blackstone M. E., Miller R. S., Hodgson A. J., Cooper S. S., Blackhurst D. W., Stein M. A. Lowering hospital charges in the trauma intensive care unit while maintaining quality of care by increasing resident and attending physician awareness. J Trauma. 1995 Dec;39(6):1041–1044. doi: 10.1097/00005373-199512000-00004. [DOI] [PubMed] [Google Scholar]
- Block S., Maier W., Bittner R., Büchler M., Malfertheiner P., Beger H. G. Identification of pancreas necrosis in severe acute pancreatitis: imaging procedures versus clinical staging. Gut. 1986 Sep;27(9):1035–1042. doi: 10.1136/gut.27.9.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke J. B. Incidence and mortality of acute pancreatitis. Br Med J. 1977 Dec 24;2(6103):1668–1669. doi: 10.1136/bmj.2.6103.1668-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley E. L., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993 May;128(5):586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- Büchler M., Malfertheiner P., Uhl W., Schölmerich J., Stöckmann F., Adler G., Gaus W., Rolle K., Beger H. G. Gabexate mesilate in human acute pancreatitis. German Pancreatitis Study Group. Gastroenterology. 1993 Apr;104(4):1165–1170. doi: 10.1016/0016-5085(93)90288-n. [DOI] [PubMed] [Google Scholar]
- Calleja G. A., Barkin J. S. Acute pancreatitis. Med Clin North Am. 1993 Sep;77(5):1037–1056. doi: 10.1016/s0025-7125(16)30209-7. [DOI] [PubMed] [Google Scholar]
- Carter D. C. Acute pancreatitis: the value of life. Br J Surg. 1993 Dec;80(12):1499–1500. doi: 10.1002/bjs.1800801202. [DOI] [PubMed] [Google Scholar]
- Cavallini G., Tittobello A., Frulloni L., Masci E., Mariana A., Di Francesco V. Gabexate for the prevention of pancreatic damage related to endoscopic retrograde cholangiopancreatography. Gabexate in digestive endoscopy--Italian Group. N Engl J Med. 1996 Sep 26;335(13):919–923. doi: 10.1056/NEJM199609263351302. [DOI] [PubMed] [Google Scholar]
- Chalfin D. B., Cohen I. L., Lambrinos J. The economics and cost-effectiveness of critical care medicine. Intensive Care Med. 1995 Nov;21(11):952–961. doi: 10.1007/BF01712339. [DOI] [PubMed] [Google Scholar]
- Corfield A. P., Cooper M. J., Williamson R. C. Acute pancreatitis: a lethal disease of increasing incidence. Gut. 1985 Jul;26(7):724–729. doi: 10.1136/gut.26.7.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield A. P., Cooper M. J., Williamson R. C., Mayer A. D., McMahon M. J., Dickson A. P., Shearer M. G., Imrie C. W. Prediction of severity in acute pancreatitis: prospective comparison of three prognostic indices. Lancet. 1985 Aug 24;2(8452):403–407. doi: 10.1016/s0140-6736(85)92733-3. [DOI] [PubMed] [Google Scholar]
- Cullen D. J., Civetta J. M., Briggs B. A., Ferrara L. C. Therapeutic intervention scoring system: a method for quantitative comparison of patient care. Crit Care Med. 1974 Mar-Apr;2(2):57–60. [PubMed] [Google Scholar]
- Curley P., Nestor M., Collins K., Saporoschetz I., Mendez M., Mannick J. A., Rodrick M. L. Decreased interleukin-2 production in murine acute pancreatitis: potential for immunomodulation. Gastroenterology. 1996 Feb;110(2):583–588. doi: 10.1053/gast.1996.v110.pm8566607. [DOI] [PubMed] [Google Scholar]
- Davies M. G., Hagen P. O. Systemic inflammatory response syndrome. Br J Surg. 1997 Jul;84(7):920–935. doi: 10.1002/bjs.1800840707. [DOI] [PubMed] [Google Scholar]
- Doepel M., Eriksson J., Halme L., Kumpulainen T., Höckerstedt K. Good long-term results in patients surviving severe acute pancreatitis. Br J Surg. 1993 Dec;80(12):1583–1586. doi: 10.1002/bjs.1800801229. [DOI] [PubMed] [Google Scholar]
- Domínguez-Muñoz J. E., Carballo F., García M. J., de Diego J. M., Rábago L., Simón M. A., de la Morena J. Clinical usefulness of polymorphonuclear elastase in predicting the severity of acute pancreatitis: results of a multicentre study. Br J Surg. 1991 Oct;78(10):1230–1234. doi: 10.1002/bjs.1800781027. [DOI] [PubMed] [Google Scholar]
- Emanuelli G., Montrucchio G., Dughera L., Gaia E., Lupia E., Battaglia E., De Martino A., De Giuli P., Gubetta L., Camussi G. Role of platelet activating factor in acute pancreatitis induced by lipopolysaccharides in rabbits. Eur J Pharmacol. 1994 Aug 22;261(3):265–272. doi: 10.1016/0014-2999(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Exley A. R., Leese T., Holliday M. P., Swann R. A., Cohen J. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut. 1992 Aug;33(8):1126–1128. doi: 10.1136/gut.33.8.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S. T., Lai E. C., Mok F. P., Lo C. M., Zheng S. S., Wong J. Early treatment of acute biliary pancreatitis by endoscopic papillotomy. N Engl J Med. 1993 Jan 28;328(4):228–232. doi: 10.1056/NEJM199301283280402. [DOI] [PubMed] [Google Scholar]
- Fenton-Lee D., Imrie C. W. Pancreatic necrosis: assessment of outcome related to quality of life and cost of management. Br J Surg. 1993 Dec;80(12):1579–1582. doi: 10.1002/bjs.1800801228. [DOI] [PubMed] [Google Scholar]
- Formela L. J., Wood L. M., Whittaker M., Kingsnorth A. N. Amelioration of experimental acute pancreatitis with a potent platelet-activating factor antagonist. Br J Surg. 1994 Dec;81(12):1783–1785. doi: 10.1002/bjs.1800811224. [DOI] [PubMed] [Google Scholar]
- Forsmark C. E., Toskes P. P. Acute pancreatitis. Medical management. Crit Care Clin. 1995 Apr;11(2):295–309. [PubMed] [Google Scholar]
- Funnell I. C., Bornman P. C., Weakley S. P., Terblanche J., Marks I. N. Obesity: an important prognostic factor in acute pancreatitis. Br J Surg. 1993 Apr;80(4):484–486. doi: 10.1002/bjs.1800800426. [DOI] [PubMed] [Google Scholar]
- Fölsch U. R., Nitsche R., Lüdtke R., Hilgers R. A., Creutzfeldt W. Early ERCP and papillotomy compared with conservative treatment for acute biliary pancreatitis. The German Study Group on Acute Biliary Pancreatitis. N Engl J Med. 1997 Jan 23;336(4):237–242. doi: 10.1056/NEJM199701233360401. [DOI] [PubMed] [Google Scholar]
- Gudgeon A. M., Heath D. I., Hurley P., Jehanli A., Patel G., Wilson C., Shenkin A., Austen B. M., Imrie C. W., Hermon-Taylor J. Trypsinogen activation peptides assay in the early prediction of severity of acute pancreatitis. Lancet. 1990 Jan 6;335(8680):4–8. doi: 10.1016/0140-6736(90)90135-r. [DOI] [PubMed] [Google Scholar]
- Gyldmark M. A review of cost studies of intensive care units: problems with the cost concept. Crit Care Med. 1995 May;23(5):964–972. doi: 10.1097/00003246-199505000-00028. [DOI] [PubMed] [Google Scholar]
- Heath D. I., Cruickshank A., Gudgeon M., Jehanli A., Shenkin A., Imrie C. W. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut. 1993 Jan;34(1):41–45. doi: 10.1136/gut.34.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedström J., Sainio V., Kemppainen E., Haapiainen R., Kivilaakso E., Schröder T., Leinonen J., Stenman U. H. Serum complex of trypsin 2 and alpha 1 antitrypsin as diagnostic and prognostic marker of acute pancreatitis: clinical study in consecutive patients. BMJ. 1996 Aug 10;313(7053):333–337. doi: 10.1136/bmj.313.7053.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. R., Wagner D. S. Gallstone pancreatitis: a prospective randomized trial of the timing of surgery. Surgery. 1988 Oct;104(4):600–605. [PubMed] [Google Scholar]
- Kingsnorth A. N., Galloway S. W., Formela L. J. Randomized, double-blind phase II trial of Lexipafant, a platelet-activating factor antagonist, in human acute pancreatitis. Br J Surg. 1995 Oct;82(10):1414–1420. doi: 10.1002/bjs.1800821039. [DOI] [PubMed] [Google Scholar]
- Kivilaakso E., Lempinen M., Mäkeläinen A., Nikki P., Schröder T. Pancreatic resection versus peritoneal lavation for acute fulminant pancreatitis. A randomized prospective study. Ann Surg. 1984 Apr;199(4):426–431. doi: 10.1097/00000658-198404000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus W. A., Draper E. A., Wagner D. P., Zimmerman J. E. APACHE II: a severity of disease classification system. Crit Care Med. 1985 Oct;13(10):818–829. [PubMed] [Google Scholar]
- Lankisch P. G., Schirren C. A. Increased body weight as a prognostic parameter for complications in the course of acute pancreatitis. Pancreas. 1990 Sep;5(5):626–629. doi: 10.1097/00006676-199009000-00021. [DOI] [PubMed] [Google Scholar]
- Lankisch P. G., Schirren C. A., Kunze E. Undetected fatal acute pancreatitis: why is the disease so frequently overlooked? Am J Gastroenterol. 1991 Mar;86(3):322–326. [PubMed] [Google Scholar]
- Larvin M., McMahon M. J. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989 Jul 22;2(8656):201–205. doi: 10.1016/s0140-6736(89)90381-4. [DOI] [PubMed] [Google Scholar]
- Leach S. D., Gorelick F. S., Modlin I. M. New perspectives on acute pancreatitis. Scand J Gastroenterol Suppl. 1992;192:29–38. doi: 10.3109/00365529209095976. [DOI] [PubMed] [Google Scholar]
- Leese T., Holliday M., Heath D., Hall A. W., Bell P. R. Multicentre clinical trial of low volume fresh frozen plasma therapy in acute pancreatitis. Br J Surg. 1987 Oct;74(10):907–911. doi: 10.1002/bjs.1800741012. [DOI] [PubMed] [Google Scholar]
- Leese T., Shaw D. Comparison of three Glasgow multifactor prognostic scoring systems in acute pancreatitis. Br J Surg. 1988 May;75(5):460–462. doi: 10.1002/bjs.1800750519. [DOI] [PubMed] [Google Scholar]
- Livingston D. H., Deitch E. A. Multiple organ failure: a common problem in surgical intensive care unit patients. Ann Med. 1995 Feb;27(1):13–20. doi: 10.3109/07853899509031931. [DOI] [PubMed] [Google Scholar]
- London N. J., Leese T., Lavelle J. M., Miles K., West K. P., Watkin D. F., Fossard D. P. Rapid-bolus contrast-enhanced dynamic computed tomography in acute pancreatitis: a prospective study. Br J Surg. 1991 Dec;78(12):1452–1456. doi: 10.1002/bjs.1800781216. [DOI] [PubMed] [Google Scholar]
- London N. J., Neoptolemos J. P., Lavelle J., Bailey I., James D. Contrast-enhanced abdominal computed tomography scanning and prediction of severity of acute pancreatitis: a prospective study. Br J Surg. 1989 Mar;76(3):268–272. doi: 10.1002/bjs.1800760317. [DOI] [PubMed] [Google Scholar]
- Luiten E. J., Hop W. C., Lange J. F., Bruining H. A. Controlled clinical trial of selective decontamination for the treatment of severe acute pancreatitis. Ann Surg. 1995 Jul;222(1):57–65. doi: 10.1097/00000658-199507000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A. D., McMahon M. J., Corfield A. P., Cooper M. J., Williamson R. C., Dickson A. P., Shearer M. G., Imrie C. W. Controlled clinical trial of peritoneal lavage for the treatment of severe acute pancreatitis. N Engl J Med. 1985 Feb 14;312(7):399–404. doi: 10.1056/NEJM198502143120703. [DOI] [PubMed] [Google Scholar]
- McFadden D. W. Organ failure and multiple organ system failure in pancreatitis. Pancreas. 1991;6 (Suppl 1):S37–S43. doi: 10.1097/00006676-199101001-00007. [DOI] [PubMed] [Google Scholar]
- Messori A., Rampazzo R., Scroccaro G., Olivato R., Bassi C., Falconi M., Pederzoli P., Martini N. Effectiveness of gabexate mesilate in acute pancreatitis. A metaanalysis. Dig Dis Sci. 1995 Apr;40(4):734–738. doi: 10.1007/BF02064970. [DOI] [PubMed] [Google Scholar]
- Mithöfer K., Fernández-del Castillo C., Ferraro M. J., Lewandrowski K., Rattner D. W., Warshaw A. L. Antibiotic treatment improves survival in experimental acute necrotizing pancreatitis. Gastroenterology. 1996 Jan;110(1):232–240. doi: 10.1053/gast.1996.v110.pm8536862. [DOI] [PubMed] [Google Scholar]
- Neoptolemos J. P., Carr-Locke D. L., London N. J., Bailey I. A., James D., Fossard D. P. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988 Oct 29;2(8618):979–983. doi: 10.1016/s0140-6736(88)90740-4. [DOI] [PubMed] [Google Scholar]
- Neoptolemos J. P., London N. J., Carr-Locke D. L. Assessment of main pancreatic duct integrity by endoscopic retrograde pancreatography in patients with acute pancreatitis. Br J Surg. 1993 Jan;80(1):94–99. doi: 10.1002/bjs.1800800131. [DOI] [PubMed] [Google Scholar]
- Niederau C., Schulz H. U. Current conservative treatment of acute pancreatitis: evidence from animal and human studies. Hepatogastroenterology. 1993 Dec;40(6):538–549. [PubMed] [Google Scholar]
- Poston G. J., Williamson R. C. Surgical management of acute pancreatitis. Br J Surg. 1990 Jan;77(1):5–12. doi: 10.1002/bjs.1800770104. [DOI] [PubMed] [Google Scholar]
- Renner I. G., Savage W. T., 3rd, Pantoja J. L., Renner V. J. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985 Oct;30(10):1005–1018. doi: 10.1007/BF01308298. [DOI] [PubMed] [Google Scholar]
- Reynaert M. S., Dugernier T., Kestens P. J. Current therapeutic strategies in severe acute pancreatitis. Intensive Care Med. 1990;16(6):352–362. doi: 10.1007/BF01735172. [DOI] [PubMed] [Google Scholar]
- Sainio V., Kemppainen E., Puolakkainen P., Taavitsainen M., Kivisaari L., Valtonen V., Haapiainen R., Schröder T., Kivilaakso E. Early antibiotic treatment in acute necrotising pancreatitis. Lancet. 1995 Sep 9;346(8976):663–667. doi: 10.1016/s0140-6736(95)92280-6. [DOI] [PubMed] [Google Scholar]
- Talamini G., Bassi C., Falconi M., Frulloni L., Di Francesco V., Vaona B., Bovo P., Rigo L., Castagnini A., Angelini G. Cigarette smoking: an independent risk factor in alcoholic pancreatitis. Pancreas. 1996 Mar;12(2):131–137. [PubMed] [Google Scholar]
- Tanaka N., Murata A., Uda K., Toda H., Kato T., Hayashida H., Matsuura N., Mori T. Interleukin-1 receptor antagonist modifies the changes in vital organs induced by acute necrotizing pancreatitis in a rat experimental model. Crit Care Med. 1995 May;23(5):901–908. doi: 10.1097/00003246-199505000-00019. [DOI] [PubMed] [Google Scholar]
- Trapnell J. E. The natural history and prognosis of acute pancreatitis. Ann R Coll Surg Engl. 1966 May;38(5):265–287. [PMC free article] [PubMed] [Google Scholar]
- Venable M. E., Zimmerman G. A., McIntyre T. M., Prescott S. M. Platelet-activating factor: a phospholipid autacoid with diverse actions. J Lipid Res. 1993 May;34(5):691–702. [PubMed] [Google Scholar]
- Ward J. B., Petersen O. H., Jenkins S. A., Sutton R. Is an elevated concentration of acinar cytosolic free ionised calcium the trigger for acute pancreatitis? Lancet. 1995 Oct 14;346(8981):1016–1019. doi: 10.1016/s0140-6736(95)91695-4. [DOI] [PubMed] [Google Scholar]
- Ward J. B., Sutton R., Jenkins S. A., Petersen O. H. Progressive disruption of acinar cell calcium signaling is an early feature of cerulein-induced pancreatitis in mice. Gastroenterology. 1996 Aug;111(2):481–491. doi: 10.1053/gast.1996.v111.pm8690215. [DOI] [PubMed] [Google Scholar]
- Whitcomb D. C., Gorry M. C., Preston R. A., Furey W., Sossenheimer M. J., Ulrich C. D., Martin S. P., Gates L. K., Jr, Amann S. T., Toskes P. P. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996 Oct;14(2):141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- Wilson P. G., Manji M., Neoptolemos J. P. Acute pancreatitis as a model of sepsis. J Antimicrob Chemother. 1998 Jan;41 (Suppl A):51–63. doi: 10.1093/jac/41.suppl_1.51. [DOI] [PubMed] [Google Scholar]
- Winslet M., Hall C., London N. J., Neoptolemos J. P. Relation of diagnostic serum amylase levels to aetiology and severity of acute pancreatitis. Gut. 1992 Jul;33(7):982–986. doi: 10.1136/gut.33.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaux A. C., Palmer K. R., Carter D. C. Factors influencing morbidity and mortality in acute pancreatitis; an analysis of 279 cases. Gut. 1995 Jul;37(1):121–126. doi: 10.1136/gut.37.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]