Abstract
Background/aims—The gene promoter for the intercellular adhesion molecule ICAM-1 possesses binding sites for several transcriptional factors, including nuclear factor κB (NF-κB). The role of NF-κB in ICAM-1 gene regulation was therefore examined by using different proteasome inhibitors in tumour necrosis factor α (TNF-α) stimulated IEC-6 rat intestinal epithelial cells. Methods—ICAM-1 expression was analysed by enzyme linked immunosorbent assay (ELISA), reverse transcriptase polymerase chain reaction, and immunohistochemistry. Steady state levels of cytoplasmic IκB protein were evaluated by western blot, and nuclear translocation of NF-κB was determined by electrophoretic mobility shift assay and immunofluorescence staining. Cell adhesion was assayed by measuring the binding of fluorescence labelled MOLT-4 cells. Results—TNF-α induced ICAM-1 mRNA and protein expression in IEC-6 cells, which was followed by increased adhesion of MOLT-4 lymphocytes. Blocking TNF-α induced IκBα degradation with proteasome inhibitors reduced TNF-α induced NF-κB activation and ICAM-1 gene induction and notably decreased MOLT-4 cell adhesion without affecting Jun N-terminal kinase (JNK/SAPK) activity or de novo protein synthesis. Conclusion—TNF-α induction of ICAM-1 expression is mediated by the transcription factor NF-κB and can be inhibited by blocking IκBα degradation. Thus the IκB/NF-κB system is a promising target for pharmacological modulation of the expression of adhesion molecules and other inflammatory genes in the intestine.
Keywords: adhesion molecules; ICAM-1; cytokines; tumour necrosis factor α; intestinal inflammation; NF-κB.
Full Text
The Full Text of this article is available as a PDF (232.2 KB).
Figure 1 .
Effect of tumour necrosis factor α (TNF-α) stimulation and selective proteasome blockade on steady state IκBα concentrations (A), NF-κB binding activity (B), and RelA (p65) subcellular localisation (C). (A) Cells were pretreated with MG-132 (5 µg/ml; left panel), ALLN (50 µg/ml; right panel), or medium alone for 30 minutes and then stimulated for 0 to 60 minutes with TNF-α (10 ng/ml). Total protein was extracted and 20 µg subjected to SDS/PAGE followed by immunoblotting of IκBα using the ECL technique as described in the Methods section. Note that the IκBα standard used as a control contains seven extra amino acids (IκB-tag) compared with the endogenous epithelial IκBα. (B) Cells were treated as described above or infected for 12 hours with the Ad5IκB virus and then stimulated for 30 minutes with TNF-α (10 ng/ml). Nuclear extracts (5 µg) were tested for κB binding activity by electrophoretic mobility shift assay. Antibody supershifting is indicated by arrowheads. (C) Cells were treated as described in (A) and RelA localisation was visualised using an anti-RelA antibody followed by a rhodamine conjugated detection antibody.
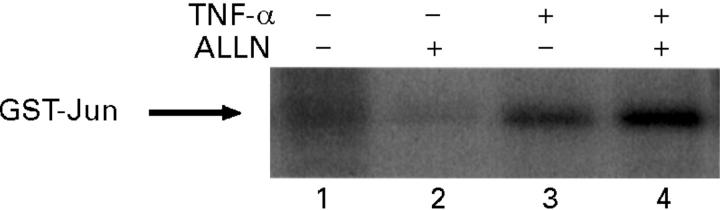
Figure 2 .
ALLN does not interfere with tumour necrosis factor α (TNF-α) induced JNK/SAPK activity in IEC-6 cells. Cells were pretreated with ALLN (50 µg/ml) or medium alone for 30 minutes and then stimulated with TNF-α (10 ng/ml) for 30 minutes. Phosphorylated glutathione S-transferase (GST)-c-Jun was visualised after protein fractionation using SDS/PAGE (12.5% gel) and quantified using PhosphorImager analysis. Coomassie staining was used to confirm equal protein loading. A representative result of three independent experiments is shown.
Figure 3 .
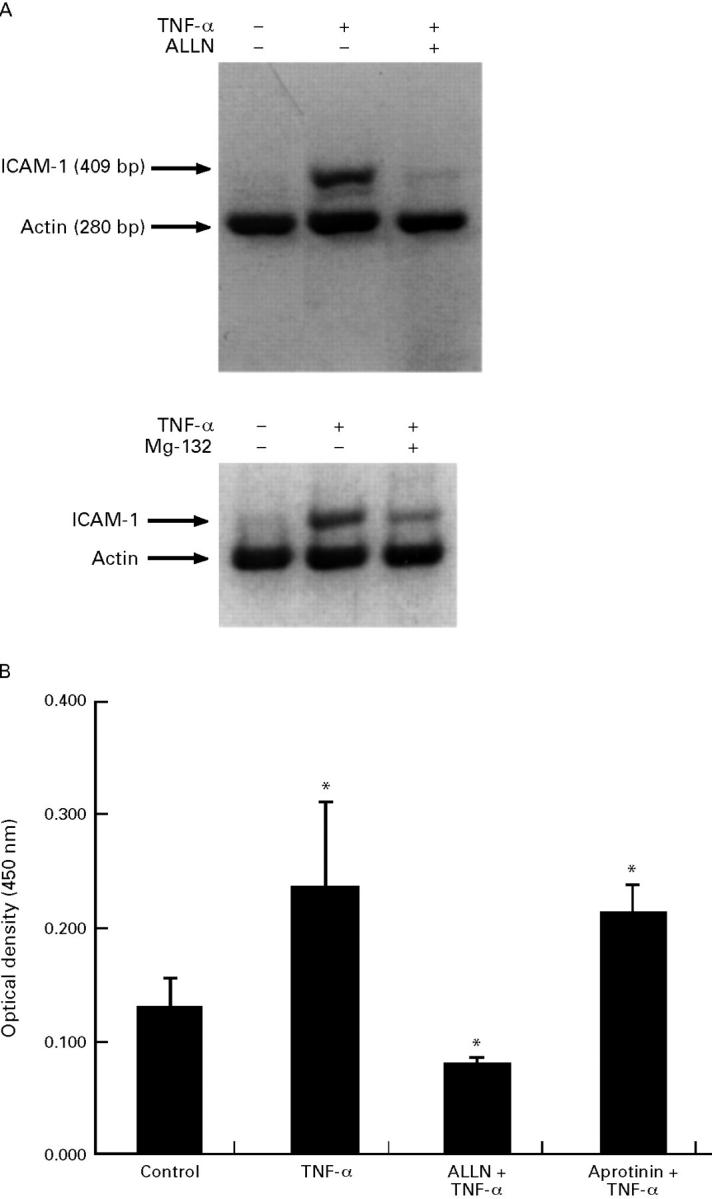
Inhibition of ICAM-1 gene expression by proteasome inhibitors in IEC-6 cells as measured by RT-PCR (A) and ELISA (B) techniques. (A) Cells were pretreated with ALLN (50 µg/ml; upper panel), MG-132 (5 µg/ml; lower panel), or medium alone for 30 minutes and then stimulated with tumour necrosis factor α (TNF-α) (10 ng/ml) or medium alone for four hours. Total RNA was extracted, reverse transcribed, and amplified using specific ICAM-1 or actin primers. PCR products were run on a 2% agarose gel and stained with ethidium bromide. These results are representative of three different experiments. (B) Cells were stimulated with TNF-α (10 ng/ml) or medium alone for 16 hours in the presence or absence of ALLN (50 µg/ml) or aprotinin (10 µg/ml). Immunoreactive ICAM-1 protein synthesis was measured as described in the Methods section. Data represent mean (SE) from four samples. *p<0.05 compared with control samples. This result is representative of three different experiments.
Figure 4 .
Tumour necrosis factor α (TNF-α) induces ICAM-1 protein systhesis in IEC-6 cells as measured by immunohistochemical analysis. Cells were grown on glass coverslips and then stimulated with TNF-α (10 ng/ml) (B) or cultured in medium alone (A) for 16 hours. The cells were stained for ICAM-1 using anti-ICAM-1 antibody as described in the Methods section. Similar results were obtained in two other experiments.
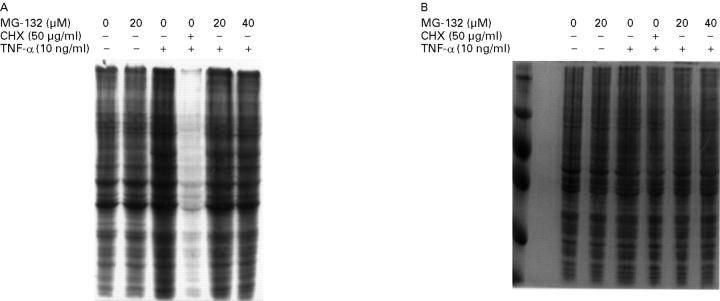
Figure 5 .
Effect of MG-132 on IEC-6 cellular protein synthesis. Cells were starved for 60 minutes in medium without methionine and then labelled for 12 hours with 150 µCi [35S]methionine in the presence or absence of MG-132 or cycloheximide (CHX). Total protein was extracted and 20 µg subjected to SDS/PAGE. (A) Gel exposed to autoradiography or (B) stained with Coomassie blue as described in the Methods section. Similar results were observed in two other independent experiments.
Figure 6 .
ALLN blocks the tumour necrosis factor α (TNF-α) mediated adhesion of IEC-6 to MOLT-4 lymphocytes. Fluorescence labelled MOLT-4 cells were added to TNF-α stimulated IEC-6 cells pretreated with ALLN (50 µg/ml), aprotinin (10 µg/ml), or medium alone. Fluorescence emission intensity in cell lysates after washing was measured as described in the Methods section. Similar inhibition was observed in two other independent experiments.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Beauparlant P., Hiscott J. Biological and biochemical inhibitors of the NF-kappa B/Rel proteins and cytokine synthesis. Cytokine Growth Factor Rev. 1996 Aug;7(2):175–190. doi: 10.1016/1359-6101(96)00020-2. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Finco T. S., Nantermet P. V., Baldwin A. S., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993 Jun;13(6):3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. F., Kornbrust D., Henry S., Stecker K., Howard R., Cooper S., Dutson S., Hall W., Jacoby H. I. An ICAM-1 antisense oligonucleotide prevents and reverses dextran sulfate sodium-induced colitis in mice. J Pharmacol Exp Ther. 1997 Feb;280(2):988–1000. [PubMed] [Google Scholar]
- Chen Z. J., Parent L., Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996 Mar 22;84(6):853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- Cobb R. R., Felts K. A., Parry G. C., Mackman N. Proteasome inhibitors block VCAM-1 and ICAM-1 gene expression in endothelial cells without affecting nuclear translocation of nuclear factor-kappa B. Eur J Immunol. 1996 Apr;26(4):839–845. doi: 10.1002/eji.1830260417. [DOI] [PubMed] [Google Scholar]
- Colgan S. P., Parkos C. A., Delp C., Arnaout M. A., Madara J. L. Neutrophil migration across cultured intestinal epithelial monolayers is modulated by epithelial exposure to IFN-gamma in a highly polarized fashion. J Cell Biol. 1993 Feb;120(3):785–798. doi: 10.1083/jcb.120.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Read M. A., Neish A. S., Whitley M. Z., Thanos D., Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995 Jul;9(10):899–909. [PubMed] [Google Scholar]
- Dippold W., Wittig B., Schwaeble W., Mayet W., Meyer zum Büschenfelde K. H. Expression of intercellular adhesion molecule 1 (ICAM-1, CD54) in colonic epithelial cells. Gut. 1993 Nov;34(11):1593–1597. doi: 10.1136/gut.34.11.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongworth D. W., Gotch F. M., Hildreth J. E., Morris A., McMichael A. J. Effects of monoclonal antibodies to the alpha and beta chains of the human lymphocyte function-associated (H-LFA-1) antigen on T lymphocyte functions. Eur J Immunol. 1985 Sep;15(9):888–892. doi: 10.1002/eji.1830150905. [DOI] [PubMed] [Google Scholar]
- Dougherty G. J., Murdoch S., Hogg N. The function of human intercellular adhesion molecule-1 (ICAM-1) in the generation of an immune response. Eur J Immunol. 1988 Jan;18(1):35–39. doi: 10.1002/eji.1830180107. [DOI] [PubMed] [Google Scholar]
- Dérijard B., Hibi M., Wu I. H., Barrett T., Su B., Deng T., Karin M., Davis R. J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994 Mar 25;76(6):1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Fan C. M., Maniatis T. Generation of p50 subunit of NF-kappa B by processing of p105 through an ATP-dependent pathway. Nature. 1991 Dec 5;354(6352):395–398. doi: 10.1038/354395a0. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira M. E., Banik N., Wilk S. Comparison of the effect of calpain inhibitors on two extralysosomal proteinases: the multicatalytic proteinase complex and m-calpain. J Neurochem. 1994 May;62(5):1989–1994. doi: 10.1046/j.1471-4159.1994.62051989.x. [DOI] [PubMed] [Google Scholar]
- Finco T. S., Baldwin A. S. Mechanistic aspects of NF-kappa B regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995 Sep;3(3):263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- Finco T. S., Beg A. A., Baldwin A. S., Jr Inducible phosphorylation of I kappa B alpha is not sufficient for its dissociation from NF-kappa B and is inhibited by protease inhibitors. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11884–11888. doi: 10.1073/pnas.91.25.11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Hellerbrand, Wang S. C., Tsukamoto H., Brenner D. A., Rippe R. A. Expression of intracellular adhesion molecule 1 by activated hepatic stellate cells. Hepatology. 1996 Sep;24(3):670–676. doi: 10.1002/hep.510240333. [DOI] [PubMed] [Google Scholar]
- Henkel T., Machleidt T., Alkalay I., Krönke M., Ben-Neriah Y., Baeuerle P. A. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993 Sep 9;365(6442):182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- Henninger D. D., Panés J., Eppihimer M., Russell J., Gerritsen M., Anderson D. C., Granger D. N. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol. 1997 Feb 15;158(4):1825–1832. [PubMed] [Google Scholar]
- Hibi M., Lin A., Smeal T., Minden A., Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993 Nov;7(11):2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Huang G. T., Eckmann L., Savidge T. C., Kagnoff M. F. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (ICAM)-1) expression and neutrophil adhesion. J Clin Invest. 1996 Jul 15;98(2):572–583. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobin C., Gauthier J. Differential effects of cell density on 5-lipoxygenase (5-LO), five-lipoxygenase-activating protein (FLAP) and interleukin-1 beta (IL-1 beta) expression in human neutrophils. Inflammation. 1997 Apr;21(2):235–250. doi: 10.1023/a:1027326405788. [DOI] [PubMed] [Google Scholar]
- Jobin C., Haskill S., Mayer L., Panja A., Sartor R. B. Evidence for altered regulation of I kappa B alpha degradation in human colonic epithelial cells. J Immunol. 1997 Jan 1;158(1):226–234. [PubMed] [Google Scholar]
- Jobin C., Panja A., Hellerbrand C., Iimuro Y., Didonato J., Brenner D. A., Sartor R. B. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappaB super-repressor in human intestinal epithelial cells. J Immunol. 1998 Jan 1;160(1):410–418. [PubMed] [Google Scholar]
- Kaiserlian D., Rigal D., Abello J., Revillard J. P. Expression, function and regulation of the intercellular adhesion molecule-1 (ICAM-1) on human intestinal epithelial cell lines. Eur J Immunol. 1991 Oct;21(10):2415–2421. doi: 10.1002/eji.1830211018. [DOI] [PubMed] [Google Scholar]
- Kvale D., Krajci P., Brandtzaeg P. Expression and regulation of adhesion molecules ICAM-1 (CD54) and LFA-3 (CD58) in human intestinal epithelial cell lines. Scand J Immunol. 1992 Jun;35(6):669–676. doi: 10.1111/j.1365-3083.1992.tb02973.x. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., Banerjee P., Nikolakaki E., Dai T., Rubie E. A., Ahmad M. F., Avruch J., Woodgett J. R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994 May 12;369(6476):156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Ledebur H. C., Parks T. P. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J Biol Chem. 1995 Jan 13;270(2):933–943. doi: 10.1074/jbc.270.2.933. [DOI] [PubMed] [Google Scholar]
- Li C. C., Dai R. M., Longo D. L. Inactivation of NF-kappa B inhibitor I kappa B alpha: ubiquitin-dependent proteolysis and its degradation product. Biochem Biophys Res Commun. 1995 Oct 4;215(1):292–301. doi: 10.1006/bbrc.1995.2465. [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Brown K., Siebenlist U. Activation of NF-kappa B requires proteolysis of the inhibitor I kappa B-alpha: signal-induced phosphorylation of I kappa B-alpha alone does not release active NF-kappa B. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):552–556. doi: 10.1073/pnas.92.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Patapoff T. W., Gillece-Castro B., Colgan S. P., Parkos C. A., Delp C., Mrsny R. J. 5'-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993 May;91(5):2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Gugel E. A., Dustin M. L., Springer T. A., Shaw S. Functional evidence that intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1-dependent adhesion in T cell-mediated cytotoxicity. Eur J Immunol. 1988 Apr;18(4):637–640. doi: 10.1002/eji.1830180423. [DOI] [PubMed] [Google Scholar]
- McGee D. W., Bamberg T., Vitkus S. J., McGhee J. R. A synergistic relationship between TNF-alpha, IL-1 beta, and TGF-beta 1 on IL-6 secretion by the IEC-6 intestinal epithelial cell line. Immunology. 1995 Sep;86(1):6–11. [PMC free article] [PubMed] [Google Scholar]
- McGee D. W., Beagley K. W., Aicher W. K., McGhee J. R. Transforming growth factor-beta and IL-1 beta act in synergy to enhance IL-6 secretion by the intestinal epithelial cell line, IEC-6. J Immunol. 1993 Jul 15;151(2):970–978. [PubMed] [Google Scholar]
- Miyamoto S., Maki M., Schmitt M. J., Hatanaka M., Verma I. M. Tumor necrosis factor alpha-induced phosphorylation of I kappa B alpha is a signal for its degradation but not dissociation from NF-kappa B. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12740–12744. doi: 10.1073/pnas.91.26.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Ohtani H., Watanabe Y., Fukushima K., Matsumoto T., Kitano A., Kobayashi K., Nagura H. In situ expression of the cell adhesion molecules in inflammatory bowel disease. Evidence of immunologic activation of vascular endothelial cells. Lab Invest. 1993 Jul;69(1):77–85. [PubMed] [Google Scholar]
- Neurath M. F., Pettersson S., Meyer zum Büschenfelde K. H., Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996 Sep;2(9):998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Palombella V. J., Rando O. J., Goldberg A. L., Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994 Sep 9;78(5):773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Panja A., Barone A., Mayer L. Stimulation of lamina propria lymphocytes by intestinal epithelial cells: evidence for recognition of nonclassical restriction elements. J Exp Med. 1994 Mar 1;179(3):943–950. doi: 10.1084/jem.179.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read M. A., Neish A. S., Luscinskas F. W., Palombella V. J., Maniatis T., Collins T. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity. 1995 May;2(5):493–506. doi: 10.1016/1074-7613(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Gramm C., Rothstein L., Clark K., Stein R., Dick L., Hwang D., Goldberg A. L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994 Sep 9;78(5):761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn's disease. Gastroenterol Clin North Am. 1995 Sep;24(3):475–507. [PubMed] [Google Scholar]
- Scherer D. C., Brockman J. A., Chen Z., Maniatis T., Ballard D. W. Signal-induced degradation of I kappa B alpha requires site-specific ubiquitination. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluss H. K., Barrett T., Dérijard B., Davis R. J. Signal transduction by tumor necrosis factor mediated by JNK protein kinases. Mol Cell Biol. 1994 Dec;14(12):8376–8384. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Sun S. C., Ganchi P. A., Ballard D. W., Greene W. C. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993 Mar 26;259(5103):1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. NF-kappa B: a lesson in family values. Cell. 1995 Feb 24;80(4):529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- Westwick J. K., Brenner D. A. Methods for analyzing c-Jun kinase. Methods Enzymol. 1995;255:342–359. doi: 10.1016/s0076-6879(95)55037-2. [DOI] [PubMed] [Google Scholar]
- Westwick J. K., Weitzel C., Minden A., Karin M., Brenner D. A. Tumor necrosis factor alpha stimulates AP-1 activity through prolonged activation of the c-Jun kinase. J Biol Chem. 1994 Oct 21;269(42):26396–26401. [PubMed] [Google Scholar]
- Whiteside S. T., Ernst M. K., LeBail O., Laurent-Winter C., Rice N., Israël A. N- and C-terminal sequences control degradation of MAD3/I kappa B alpha in response to inducers of NF-kappa B activity. Mol Cell Biol. 1995 Oct;15(10):5339–5345. doi: 10.1128/mcb.15.10.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]