Abstract
Background—A new subline of the senescence accelerated mouse (SAM) P1/Yit strain has been established which shows spontaneous enteric inflammation under specific pathogen free (SPF) conditions. Aims—To elucidate the pathogenesis of enteric inflammation in this new subline. Methods—The SPF and germ free (GF) SAMP1/Yit strains were used. Histological, immunological, and microbiological characterisation of the mice with enteric inflammation was performed. Results—Histologically, enteritic inflammation developed as a discontinuous lesion in the terminal ileum and caecum with the infiltration of many inflammatory cells after 10 weeks of age. The activity of myeloperoxidase, and both immunolocalisation and mRNA expression of inducible nitric oxide synthase increased in the lesion. CD3-ε positive T cells, neutrophils, and macrophages were more numerous in the inflamed mucosa of the SAMP1/Yit strain. The GF SAMP1/Yit strain did not show any inflammation in the intestinal wall, by the age of 30 weeks, and the enteritis and caecitis developed 10 weeks after the conventionalisation of the GF SAMP1/Yit strain. Conclusion—Enteric inflammation in the ileum and caecum developed in the SAMP1/Yit strain. The pathophysiological characteristics of the disease in this mouse have some similarities to those of human inflammatory bowel disease (IBD). This mouse strain should be a useful model system for elucidating the interaction between the pathogenesis of IBD and the gut microflora.
Keywords: inflammatory bowel disease; enteritis; caecitis; senescence accelerated mouse
Full Text
The Full Text of this article is available as a PDF (402.8 KB).
Figure 1 .
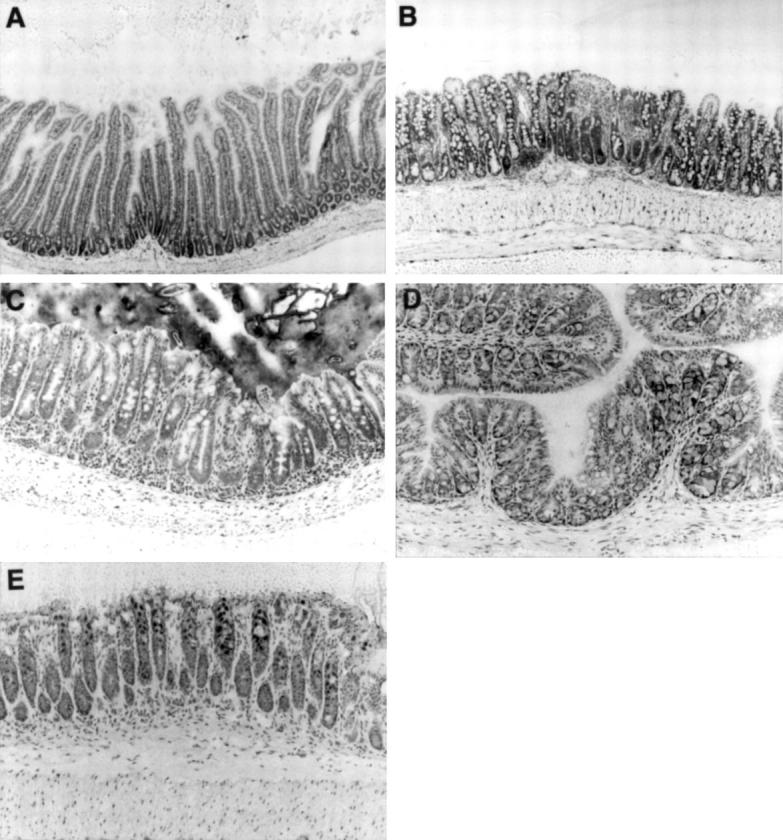
Histology of the duodenum (A), ileum (B), caecum (C), proximal colon (D), and distal colon (E) in a 20 week old SAMP1/Yit mouse. Intestinal architecture was normal in the duodenum and colon (A,D,E). Morphological changes such as villous atrophy and crypt hyperplasia were pronounced in the ileum (B). In the caecum, crypt hyperplasia, characterised by crypt elongation and increased mitotic figures, could be detected (C). Toluidine blue stain. Original magnification, ×100.
Figure 2 .
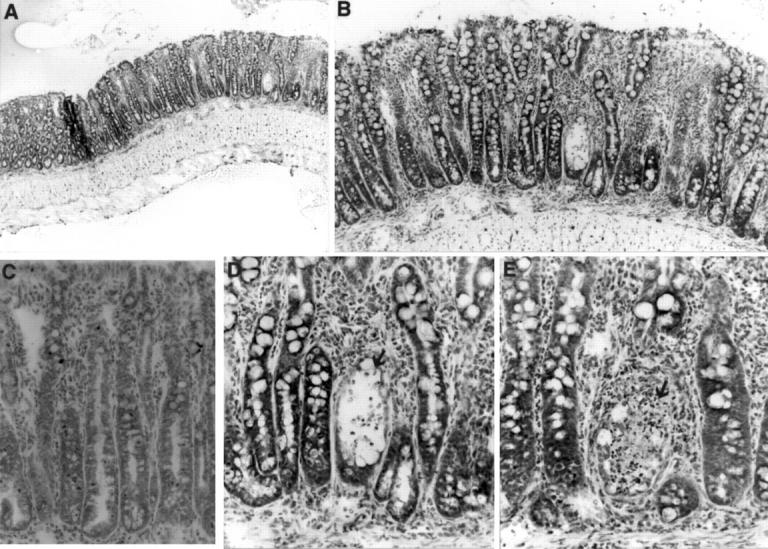
Histological analysis of the ileum of a SAMP1/Yit mouse at 20 weeks of age. (A) Low magnification photograph of inflammatory bowel disease in the ileum of a 20 week old SAMP1/Yit mouse. The intestinal walls were thickened due to the inflammation. (B) High power view of A showing severe mucosal inflammation. Crypt hyperplasia with crypt elongation, and villous atrophy were pronounced in the lesion. Inflammatory infiltrate could be observed in the LP and submucosa. (C) Enlargement of the lymphatic vessels could be seen in the LP. (D) Higher magnification of B showing crypt undergoing necrosis (arrow) with intraluminal neutrophils. Mixed inflammatory cell infiltrate in the LP predominantly consisted of macrophages, neutrophils, and lymphocytes. (E) Crypt loss (arrow) is associated with necrotic debris in crypts. Toluidine blue stain, original magnification: A, ×40; B, ×100; C, ×200; D,E ×400.
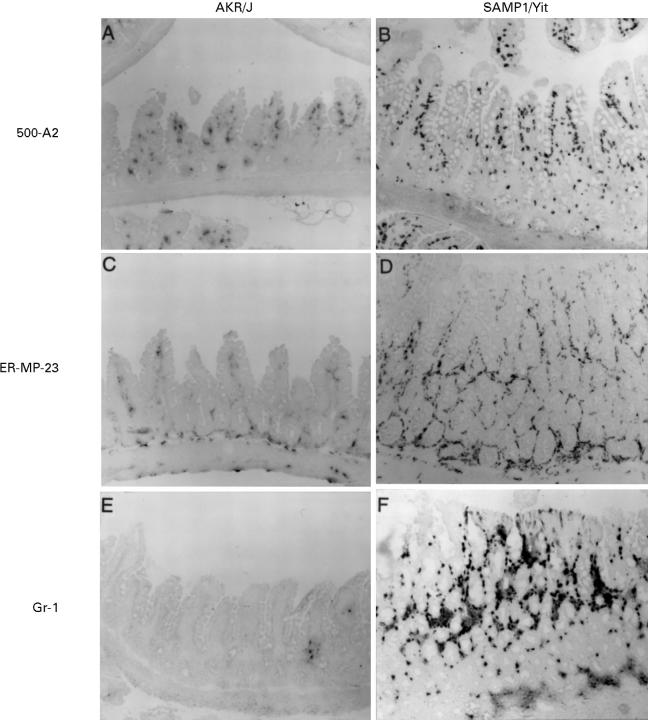
Figure 3 .
Immunohistochemical staining of the ileal mucosa. A cryostat section from IBD SAMP1/Yit and age matched control AKR/J mice was stained with monoclonal antibodies 500-A2, ER-MP-23, and Gr-1 that recognise CD3-ε, tissue macrophages, and granulocytes, respectively. CD3-ε positive cells, tissue macrophages, and granulocyes were more abundant in the IBD lesion in SAMP1/Yit (B,D,F) than in age matched control AKR/J mice (A,C,E). Original magnification, ×100.
Figure 4 .
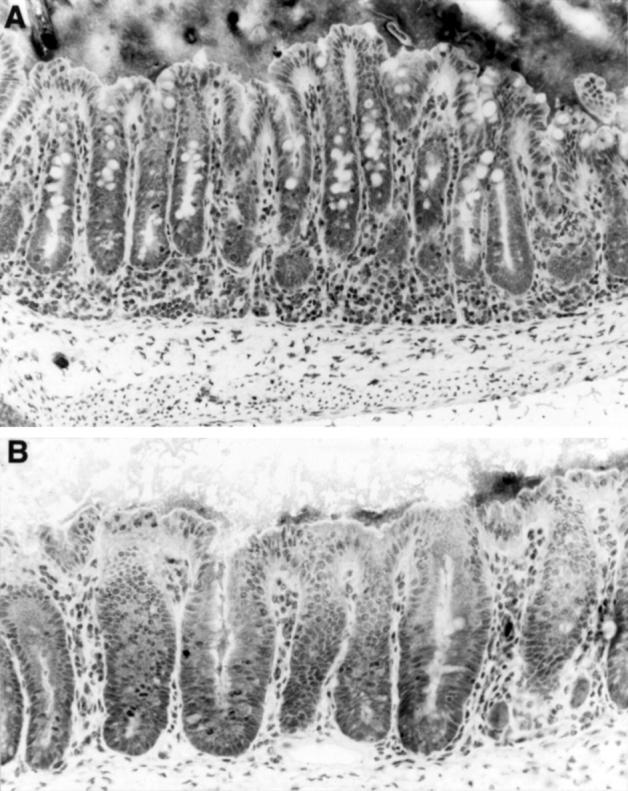
Histological analysis of the caecum of the SAMP1/Yit strain at 20 weeks of age. (A) Mild epithelial hyperplasia resulting in crypt elongation and increased mononuclear cell infiltrate in the LP. (B) Higher magnification of the caecal epithelial lesion in the SAMP1/Yit strain at age 20 weeks. Epithelial hyperplasia with increased mitotic figures could be seen. Note that there is goblet cell depletion. Toluidine blue stain. Original magnification: A, ×200; B, ×400.
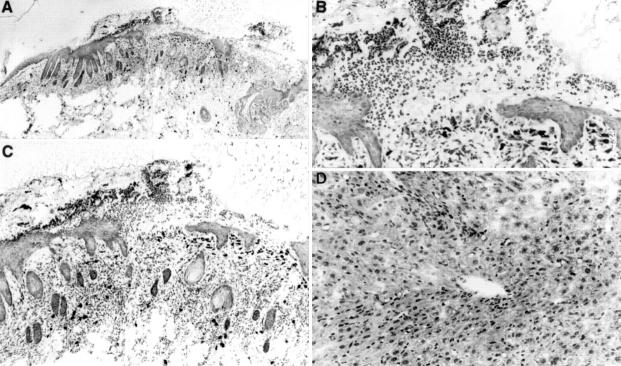
Figure 5 .
Histological analysis of the skin and liver. (A) Pathology of the dorsal skin in the SPF SAMP1/Yit strain at 20 weeks of age. Loss of the epidermis can be seen in the right side of the photograph. (B) High power view of A. Inflammatory infiltrates can be seen in the dermis. (C) Higher magnification of A. A large number of neutrophils covered the epidermal ulceration. (D) Histopathology of the liver in SPF SAMP1/Yit mice at 30 weeks of age. A focal mononuclear cell infiltrate could be detected throughout the hepatic acini. Toluidine blue stain. Original magnification: A, ×40; B, ×100; C, ×200; D, ×400.
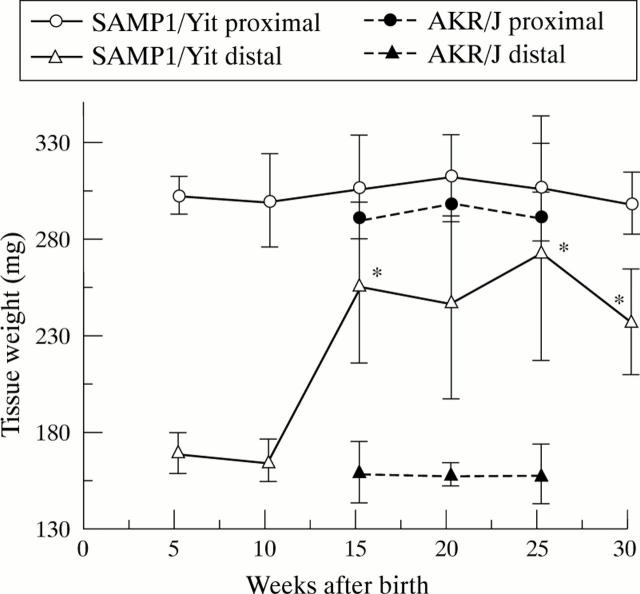
Figure 6 .
Ontogenic study of the inflammatory disease. Values are means (SD). *Significantly different (p<0.05) from the initial tissue weight.
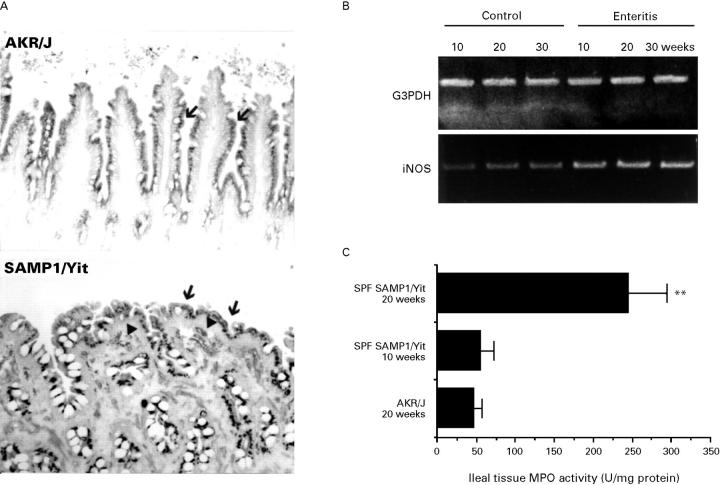
Figure 7 .
Expression of inflammatory mediators. (A) Immunostaining of iNOS in the SAMP1/Yit strain. The expression of iNOS on the epithelial cells could be seen in both SPF SAMP1/Yit and control AKR/J mice at 20 weeks of age (arrow). However, there were more iNOS expressing cells in the LP in the 20 week old SAMP1/Yit strain than in the age matched AKR/J strain (arrowhead). Original magnification ×100. (B) RT-PCR analysis of iNOS mRNA. The mRNA of iNOS increased in the ileum during disease development. (C) Analysis of tissue MPO activity. Tissue MPO activity in ileum was higher in the 20 week old SAMP1/Yit strain than in the age matched AKR/J strain. There were three mice per group. Similar results were obtained from two independent experiments. Values are means (SD). **Values significantly different (p<0.01) from control.
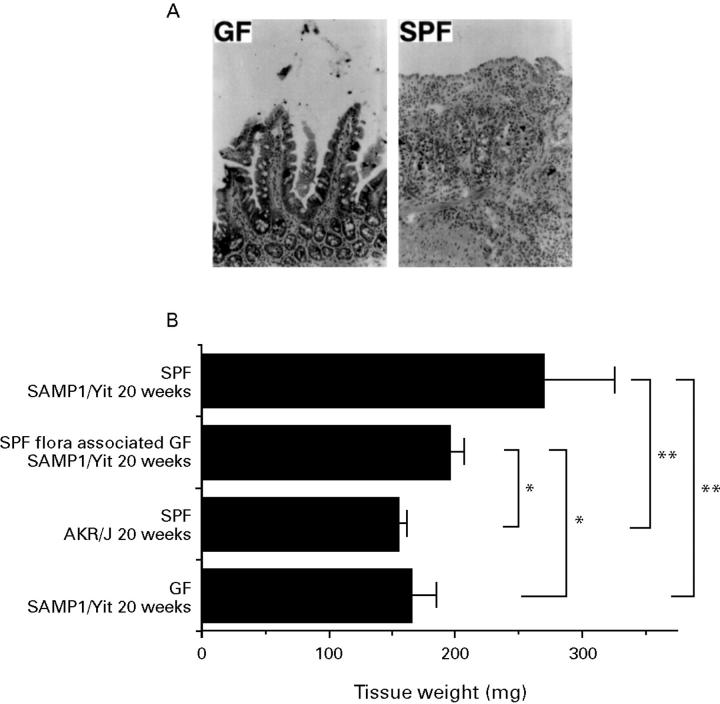
Figure 8 .
Effect of intestinal microflora on development of the disease. (A) In the GF SAMP1/Yit strain, the intestinal inflammatory disease that was seen under SPF conditions could not be detected, at least until 30 weeks of age. The intestinal architecture in GF littermates was quite normal, as in other strains of GF mice. (B) After contamination of 5 week old GF SAMP1/Yit mice with a faecal suspension from SPF AKR/J mice, the weight of the distal part of the small intestine was increased compared with that of age matched GF SAMP1/Yit mice (n=5) after 15 weeks. There was no difference in the intestinal tissue weight between GF SAMP1 and SPF AKR/J mice (p=0.4953). Values are means (SD). Values significantly different (*p<0.05; **p<0.01) from tissue weight of the GF SAMP1/Yit strain.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiko S., Grisham M. B. Spontaneous intestinal inflammation and nitric oxide metabolism in HLA-B27 transgenic rats. Gastroenterology. 1995 Jul;109(1):142–150. doi: 10.1016/0016-5085(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Berg D. J., Davidson N., Kühn R., Müller W., Menon S., Holland G., Thompson-Snipes L., Leach M. W., Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996 Aug 15;98(4):1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brkić T., Banić M., Anić B., Grabarević Z., Rotkvić I., Artuković B., Duvnjak M., Sikirić P., Troskot B., Hernandez D. E. A model of inflammatory bowel disease induced by 2,4-dinitrofluorobenzene in previously sensitized BALB-c mice. Scand J Gastroenterol. 1992;27(3):184–188. doi: 10.3109/00365529208999946. [DOI] [PubMed] [Google Scholar]
- Cahill R. J., Foltz C. J., Fox J. G., Dangler C. A., Powrie F., Schauer D. B. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997 Aug;65(8):3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K., Matsumura A., Hashimoto K., Honma A., Takeshita S., Hosokawa M., Yasuhira K., Takeda T. Isolation and characterization of senile amyloid--related antigenic substance (SASSAM) from mouse serum. Apo SASSAM is a low molecular weight apoprotein of high density lipoprotein. J Exp Med. 1983 Nov 1;158(5):1600–1614. doi: 10.1084/jem.158.5.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T., Hosono M., Hanada K., Aoike A., Kawai K., Takeda T. Immune responses in newly developed short-lived SAM mice. Selectively impaired T-helper cell activity in in vitro antibody response. Immunology. 1987 Nov;62(3):425–429. [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T., Hosono M., Higuchi K., Aoike A., Kawai K., Takeda T. Immune responses in newly developed short-lived SAM mice. I. Age-associated early decline in immune activities of cultured spleen cells. Immunology. 1987 Nov;62(3):419–423. [PMC free article] [PubMed] [Google Scholar]
- Katz J. D., Benoist C., Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995 May 26;268(5214):1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- Krawisz J. E., Sharon P., Stenson W. F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984 Dec;87(6):1344–1350. [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Setoyama H., Umesaki Y. Differential induction of major histocompatibility complex molecules on mouse intestine by bacterial colonization. Gastroenterology. 1992 Dec;103(6):1777–1782. doi: 10.1016/0016-5085(92)91434-6. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Tsuboyama T., Kasai R., Okumura H., Yamamuro T., Higuchi K., Higuchi K., Kohno A., Yonezu T., Utani A. Age-related changes in bone mass in the senescence-accelerated mouse (SAM). SAM-R/3 and SAM-P/6 as new murine models for senile osteoporosis. Am J Pathol. 1986 Nov;125(2):276–283. [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Setoyama H., Matsumoto S., Imaoka A., Nanno M., Kawaguchi M., Umesaki Y. Effects of fecal microorganisms and their chloroform-resistant variants derived from mice, rats, and humans on immunological and physiological characteristics of the intestines of ex-germfree mice. Infect Immun. 1994 Dec;62(12):5442–5446. doi: 10.1128/iai.62.12.5442-5446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990 Mar;98(3):694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Powrie F., Correa-Oliveira R., Mauze S., Coffman R. L. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994 Feb 1;179(2):589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Leach M. W., Mauze S., Caddle L. B., Coffman R. L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993 Nov;5(11):1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- Powrie F., Leach M. W., Mauze S., Menon S., Caddle L. B., Coffman R. L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994 Oct;1(7):553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Stange E. F., Fleig W. E., Rehklau E., Ditschuneit H. Cyclosporin A treatment in inflammatory bowel disease. Dig Dis Sci. 1989 Sep;34(9):1387–1392. doi: 10.1007/BF01538074. [DOI] [PubMed] [Google Scholar]
- Sundberg J. P., Elson C. O., Bedigian H., Birkenmeier E. H. Spontaneous, heritable colitis in a new substrain of C3H/HeJ mice. Gastroenterology. 1994 Dec;107(6):1726–1735. doi: 10.1016/0016-5085(94)90813-3. [DOI] [PubMed] [Google Scholar]
- Takeda T., Hosokawa M., Takeshita S., Irino M., Higuchi K., Matsushita T., Tomita Y., Yasuhira K., Hamamoto H., Shimizu K. A new murine model of accelerated senescence. Mech Ageing Dev. 1981 Oct;17(2):183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- Umesaki Y., Okada Y., Matsumoto S., Imaoka A., Setoyama H. Segmented filamentous bacteria are indigenous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol Immunol. 1995;39(8):555–562. doi: 10.1111/j.1348-0421.1995.tb02242.x. [DOI] [PubMed] [Google Scholar]
- Umesaki Y., Setoyama H., Matsumoto S., Okada Y. Expansion of alpha beta T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ-free mice and its independence from thymus. Immunology. 1993 May;79(1):32–37. [PMC free article] [PubMed] [Google Scholar]