Abstract
Background—The pathogenesis of pancreatic fibrosis is unknown. In the liver, stellate cells (vitamin A storing cells) play a significant role in the development of fibrosis. Aims—To determine whether cells resembling hepatic stellate cells are present in rat pancreas, and if so, to compare their number with the number of stellate cells in the liver, and isolate and culture these cells from rat pancreas. Methods—Liver and pancreatic sections from chow fed rats were immunostained for desmin, glial fibrillary acidic protein (GFAP), and α smooth muscle actin (α-SMA). Pancreatic stellate shaped cells were isolated using a Nycodenz gradient, cultured on plastic, and examined by phase contrast and fluorescence microscopy, and by immunostaining for desmin, GFAP, and α-SMA. Results—In both liver and pancreatic sections, stellate shaped cells were observed; these were positive for desmin and GFAP and negative for α-SMA. Pancreatic stellate shaped cells had a periacinar distribution. They comprised 3.99% of all pancreatic cells; hepatic stellate cells comprised 7.94% of all hepatic cells. The stellate shaped cells from rat pancreas grew readily in culture. Cells cultured for 24 hours had an angular appearance, contained lipid droplets manifesting positive vitamin A autofluorescence, and stained positively for desmin but negatively for α-SMA. At 48 hours, cells were positive for α-SMA. Conclusions—Cells resembling hepatic stellate cells are present in rat pancreas in a number comparable with that of stellate cells in the liver. These stellate shaped pancreatic cells can be isolated and cultured in vitro.
Keywords: pancreas; fibrosis; stellate cells
Full Text
The Full Text of this article is available as a PDF (203.6 KB).
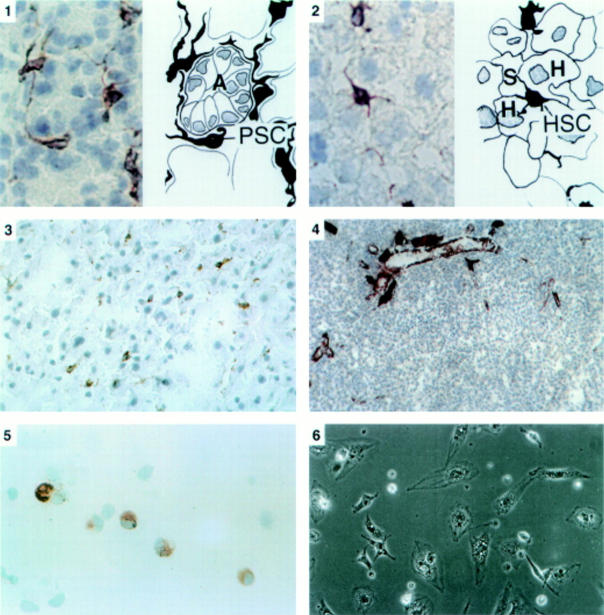
Figure 1 .
Panel 1: Immunostaining for desmin in pancreatic tissue sections (×40 objective). On the left is a photomicrograph of a section of the pancreas with a corresponding line diagram on the right. Desmin positive stellate shaped cells (PSC) were observed in the interstitium between acini (A). The cells had elongated cytoplasmic processes extending around the basal aspect of adjacent acinar cells. Panel 2: Immunostaining for desmin in liver tissue sections (×40 objective). On the left is a photomicrograph of a section of the liver with a corresponding line diagram on the right. Stellate cells (HSC) were stained brown and were located in the perisinusoidal space between hepatocytes (H) and hepatic sinusoids (S). Panel 3: Immunostaining for GFAP in pancreatic tissue sections (×25 objective). The positively stained (brown) stellate cells are seen in the same distribution as observed with the stain for desmin. Panel 4: Immunostaining for α-SMA in pancreatic tissue sections (×16 objective). No positively staining cells were found in the parenchyma of normal pancreas. Dense staining was observed in smooth muscle components of pancreatic ducts and blood vessels. Panel 5: Immunostaining for desmin in a cytospin of freshly isolated cells (×40 objective). Positive staining (brown) for desmin was observed in the cytoplasm. Panel 6: Phase contrast microscopy of cells after 24 hours in culture (×20 objective). Cells had assumed a flattened, angular appearance and showed abundant lipid droplets in the cytoplasm.
Figure 2 .
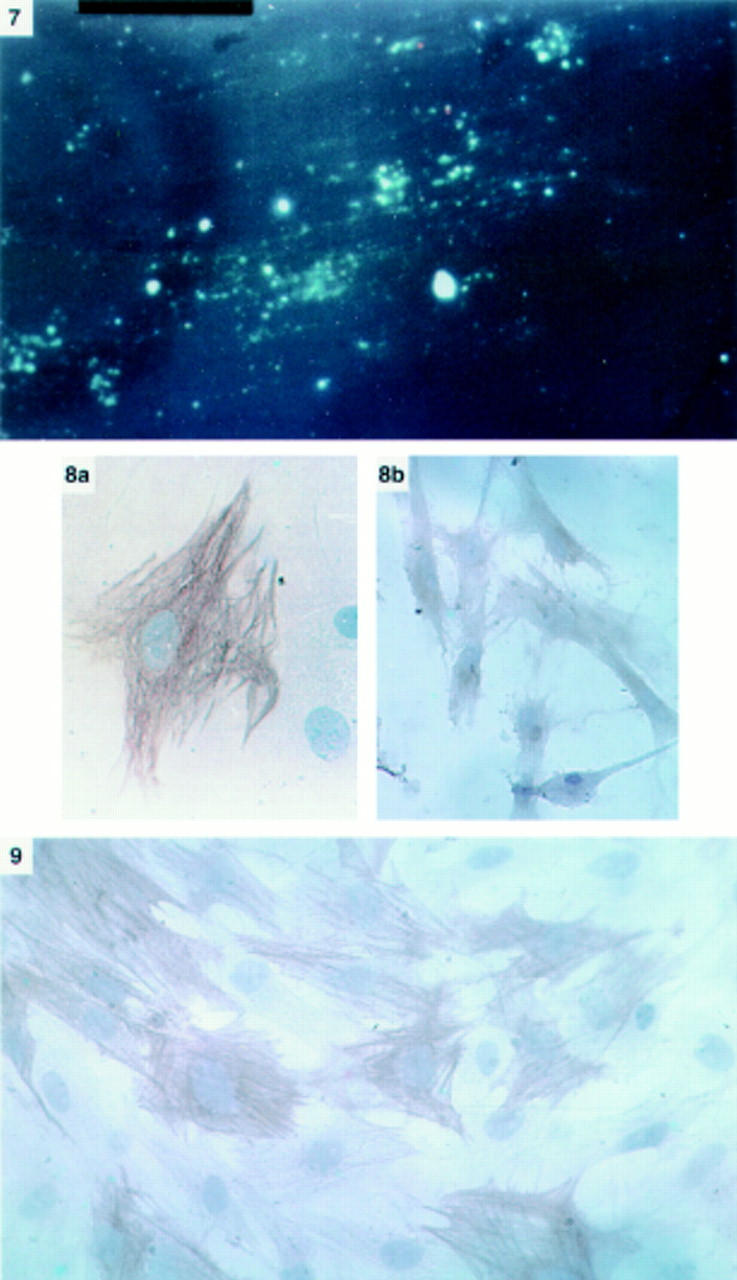
Panel 7: Vitamin A autofluorescence of cells after 24 hours in culture (×20 objective). Cells examined under ultraviolet illumination at 328 nm revealed blue-green fluorescence indicating the presence of vitamin A in the cytoplasm. Panel 8: Immunostaining for desmin (a) and GFAP (b) of pancreatic stellate shaped cells after 24 hours in culture (×40 and ×25 objective respectively). Cells were positive for both cytoskeletal markers. The high power view of the desmin positive cell clearly reveals a typical fibrillar staining pattern in the cell cytoplasm. Panel 9: Immunostaining for α-SMA of pancreatic stellate shaped cells after 48 hours in culture (×25 objective). Positive staining of cells was observed in a fibrillar pattern in the cytoplasm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altomare E., Grattagliano I., Vendemiale G., Palmieri V., Palasciano G. Acute ethanol administration induces oxidative changes in rat pancreatic tissue. Gut. 1996 May;38(5):742–746. doi: 10.1136/gut.38.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballardini G., Fallani M., Biagini G., Bianchi F. B., Pisi E. Desmin and actin in the identification of Ito cells and in monitoring their evolution to myofibroblasts in experimental liver fibrosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;56(1):45–49. doi: 10.1007/BF02890000. [DOI] [PubMed] [Google Scholar]
- Bartók I., Tóth J., Remenár E., Virágh S. Ultrastructure of the hepatic perisinusoidal cells in man and other mammalian species. Anat Rec. 1979 Aug;194(4):571–586. doi: 10.1002/ar.1091940410. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. FASEB J. 1991 Mar 1;5(3):271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- Buniatian G., Hamprecht B., Gebhardt R. Glial fibrillary acidic protein as a marker of perisinusoidal stellate cells that can distinguish between the normal and myofibroblast-like phenotypes. Biol Cell. 1996;87(1-2):65–73. [PubMed] [Google Scholar]
- Day C. P. Is necroinflammation a prerequisite for fibrogenesis? Hepatogastroenterology. 1996 Jan-Feb;43(7):104–120. [PubMed] [Google Scholar]
- Geerts A., Vrijsen R., Rauterberg J., Burt A., Schellinck P., Wisse E. In vitro differentiation of fat-storing cells parallels marked increase of collagen synthesis and secretion. J Hepatol. 1989 Jul;9(1):59–68. doi: 10.1016/0168-8278(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Gressner A. M., Bachem M. G. Molecular mechanisms of liver fibrogenesis--a homage to the role of activated fat-storing cells. Digestion. 1995;56(5):335–346. doi: 10.1159/000201257. [DOI] [PubMed] [Google Scholar]
- Gressner A. M. Cytokines and cellular crosstalk involved in the activation of fat-storing cells. J Hepatol. 1995;22(2 Suppl):28–36. [PubMed] [Google Scholar]
- Gressner A. M., Lotfi S., Gressner G., Lahme B. Identification and partial characterization of a hepatocyte-derived factor promoting proliferation of cultured fat-storing cells (parasinusoidal lipocytes). Hepatology. 1992 Nov;16(5):1250–1266. [PubMed] [Google Scholar]
- Gressner A. M. Time-related distribution profiles of sulfated glycosaminoglycans in cells, cell surfaces, and media of cultured rat liver fat-storing cells. Proc Soc Exp Biol Med. 1991 Mar;196(3):307–315. doi: 10.3181/00379727-196-43193. [DOI] [PubMed] [Google Scholar]
- Ikejiri N. The vitamin A-storing cells in the human and rat pancreas. Kurume Med J. 1990;37(2):67–81. doi: 10.2739/kurumemedj.37.67. [DOI] [PubMed] [Google Scholar]
- Parola M., Leonarduzzi G., Robino G., Albano E., Poli G., Dianzani M. U. On the role of lipid peroxidation in the pathogenesis of liver damage induced by long-standing cholestasis. Free Radic Biol Med. 1996;20(3):351–359. doi: 10.1016/0891-5849(96)02055-2. [DOI] [PubMed] [Google Scholar]
- Ramm G. A., Britton R. S., O'Neill R., Blaner W. S., Bacon B. R. Vitamin A-poor lipocytes: a novel desmin-negative lipocyte subpopulation, which can be activated to myofibroblasts. Am J Physiol. 1995 Oct;269(4 Pt 1):G532–G541. doi: 10.1152/ajpgi.1995.269.4.G532. [DOI] [PubMed] [Google Scholar]
- Rockey D. C., Boyles J. K., Gabbiani G., Friedman S. L. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992 Apr;24(2):193–203. [PubMed] [Google Scholar]
- Schäfer S., Zerbe O., Gressner A. M. The synthesis of proteoglycans in fat-storing cells of rat liver. Hepatology. 1987 Jul-Aug;7(4):680–687. doi: 10.1002/hep.1840070411. [DOI] [PubMed] [Google Scholar]
- Wake K., Motomatsu K., Senoo H. Stellate cells storing retinol in the liver of adult lamprey, Lampetra japonica. Cell Tissue Res. 1987 Aug;249(2):289–299. doi: 10.1007/BF00215511. [DOI] [PubMed] [Google Scholar]
- Watari N., Hotta Y., Mabuchi Y. Morphological studies on a vitamin A-storing cell and its complex with macrophage observed in mouse pancreatic tissues following excess vitamin A administration. Okajimas Folia Anat Jpn. 1982 Mar;58(4-6):837–858. doi: 10.2535/ofaj1936.58.4-6_837. [DOI] [PubMed] [Google Scholar]
- Yokoi Y., Namihisa T., Kuroda H., Komatsu I., Miyazaki A., Watanabe S., Usui K. Immunocytochemical detection of desmin in fat-storing cells (Ito cells). Hepatology. 1984 Jul-Aug;4(4):709–714. doi: 10.1002/hep.1840040425. [DOI] [PubMed] [Google Scholar]
- de Leeuw A. M., McCarthy S. P., Geerts A., Knook D. L. Purified rat liver fat-storing cells in culture divide and contain collagen. Hepatology. 1984 May-Jun;4(3):392–403. doi: 10.1002/hep.1840040307. [DOI] [PubMed] [Google Scholar]