Abstract
Background—Nitric oxide (NO) synthesis and inducible nitric oxide synthase (iNOS) expression are increased in colonic biopsy specimens from patients with ulcerative colitis, but the cellular source of NO production is not known. Aims—To examine the distribution of iNOS in human colonic mucosa and to explore the ability of T lymphocyte derived cytokines to regulate iNOS expression and activity in human colonic epithelial cells. Methods—iNOS expression was examined using immunohistochemistry in colonic biopsy samples from 12 patients with ulcerative colitis and three with infectious colitis and compared with 10normal controls. In vitro iNOS expression and activity were determined in HT-29 cell cultures; nitrite levels were measured using a fluorescent substrate, iNOS mRNA expression by northern blot analysis, and iNOS protein expression by western blot analysis. Results—No iNOS expression was detected (10 of 10) in non-inflamed mucosa derived from normal controls. In 11 of 12 cases of newly diagnosed ulcerative colitis, iNOS protein was expressed in the epithelial cells, while no other positive cells were found in the lamina propria. Similar iNOS labelling was found in colonic biopsy samples from patients with infectious colitis in the acute phase, but when re-examined in samples from patients in total remission, no iNOS staining was observed. Both interleukin (IL)-13 and IL-4, but not IL-10, are potent inhibitors of iNOS expression and activity induced by an optimal combination of cytokines, namely IL-1α, tumour necrosis factor α and interferon γ. Conclusions—The data suggest that the epithelium is the major source of iNOS activity in ulcerative colitis and that IL-13 and IL-4 may act as intrinsic regulators of NO generation in intestinal inflammation.
Keywords: interleukin 13; nitric oxide; inducible nitric oxide synthase; colonic epithelial cells; ulcerative colitis
Full Text
The Full Text of this article is available as a PDF (247.3 KB).
Figure 1 .

All sections were stained with anti-human inducible nitric oxide synthase (iNOS) antibody (NO-53) using avidin/biotin peroxidase. (A) Normal bowel with no detection of iNOS. Original magnification × 100. (B) Ulcerative colitis (UC) with staining of superficial section of crypt and surface epithelium. Original magnification × 100. (C) Another UC case showing similar staining; iNOS labelling is located at the apical part of the epithelial cells, in close association with neutrophil infiltration of the lamina propria (arrowheads). Original magnification × 100. (D) Acute infectious colitis with staining of the superficial section of crypt and surface epithelium. Original magnification × 100. (E) Sample from the same patient after recovery, with resolution of iNOS expression. Original magnification × 50. (F) Same section as (D) using primary antibody preabsorbed with immunogenic peptide. Original magnification × 250.
Figure 2 .
Nitrite production by HT-29 cells after treatment with cytokines. Confluent monolayers of HT-29 cells were treated with interleukin (IL)-13 (30 ng/ml), IL-4 (30 ng/ml), IL-10 (30 ng/ml), IL-1α (10 ng/ml)/interferon γ (IFN-γ) (300 U/ml) or IL-1α (10 ng/ml)/IFN-γ (300 U/ml)/tumour necrosis factor α (TNF-α) (100 ng/ml), and after 48 hours incubation at 37°C nitrite levels were determined in supernatants, using a fluorescent substrate with a 10 nM level of detection. Basal is the amount of nitrite produced by HT-29 cells in the absence of added cytokines. Results are expressed as means (SEM) from three separate experiments.
Figure 3 .
Nitrite production by HT-29 cells after treatment with interleukin (IL)-1α (10 ng/ml)/interferon γ (IFN-γ) (300 U/ml)/tumour necrosis factor (TNF-α) (100 ng/ml) in the presence of increasing concentrations of IL-13. Proinflammatory cytokines were added after one hour of pretreatment with IL-13, and nitrite levels were determined in supernatants after 24 hours incubation at 37°C. Each point represents the mean (SEM) from three separate experiments. **p<0.01, ***p<0.001 compared with positive control response (no IL-13).
Figure 4 .
Nitrite production by HT-29 cells after treatment with interleukin (IL)-1α (10 ng/ml)/interferon γ (IFN-γ) (300 U/ml)/tumour necrosis factor α (TNF-α) (100 ng/ml) in the presence of increasing concentrations of IL-4. Proinflammatory cytokines were added after one hour of pretreatment with IL-4, and nitrite levels were determined in supernatants after 24 hours incubation at 37°C. Each point represents the mean (SEM) from three separate experiments. **p<0.01, ***p<0.001 compared with positive control response (no IL-4).
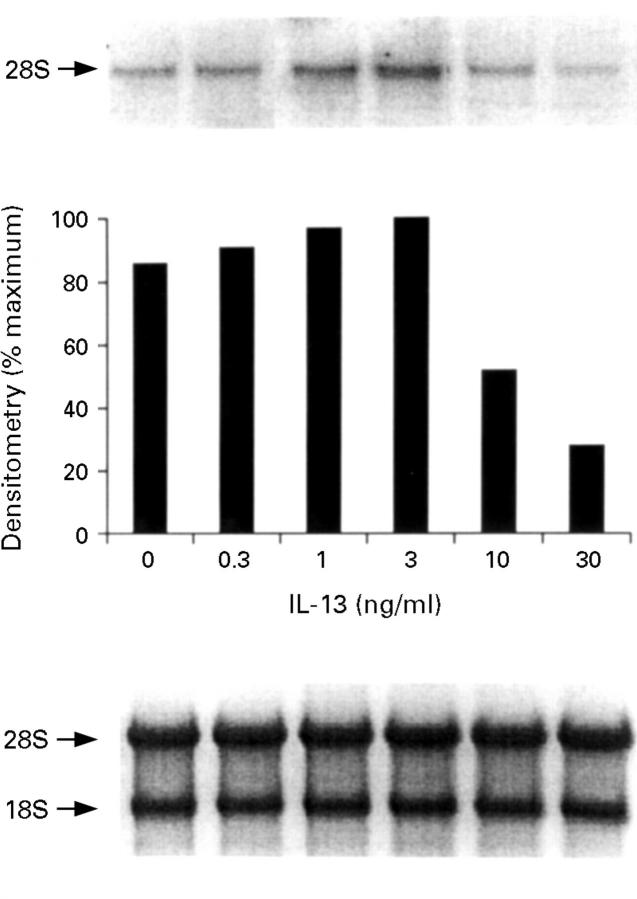
Figure 5 .
Inducible nitric oxide synthase (iNOS) mRNA expression by HT-29 cells after treatment with interleukin (IL)-1α (10 ng/ml)/interferon γ (IFN-γ) (300 U/ml)/tumour necrosis factor α (TNF-α) (100 ng/ml) in the presence of increasing concentrations of IL-13. The proinflammatory cytokines were added after one hour of pretreatment with IL-13 and incubated for 12 hours at 37°C. The top panel is the northern blot, the middle panel is the densitometry analysis of the blot, and the bottom panel the ethidium bromide stained 18S and 28S bands indicating equal loading of the lanes. This is a representative of three experiments.
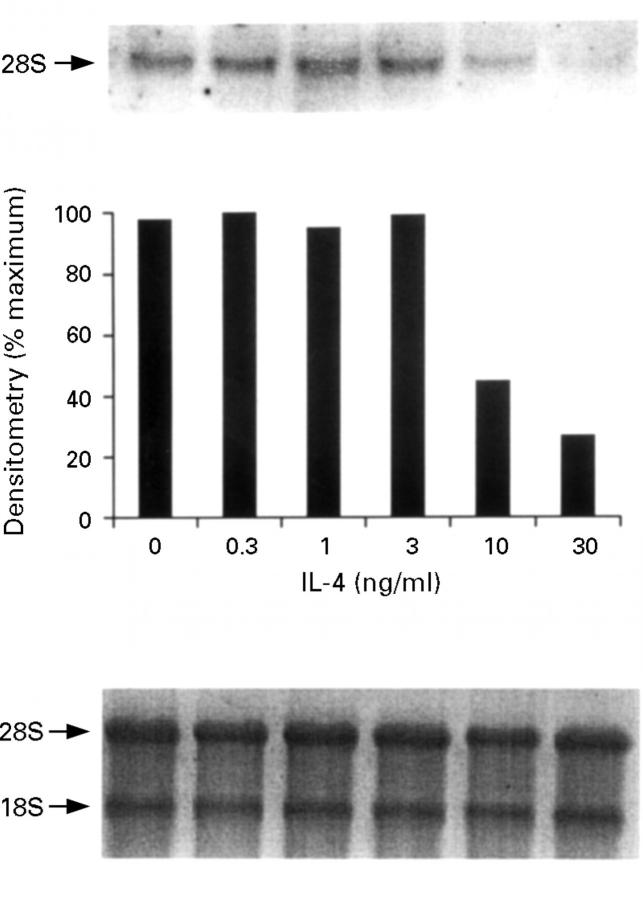
Figure 6 .
Inducible nitric oxide synthase (iNOS) mRNA expression by HT-29 cells after treatment with interleukin (IL)-1α (10 ng/ml)/interferon γ (IFN-γ) (300 U/ml)/tumour necrosis factor (TNF-α) (100 ng/ml) in the presence of increasing concentrations of IL-4. The proinflammatory cytokines were added after one hour of pretreatment with IL-4 and incubated for 12 hours at 37°C. The top panel is the northern blot, the middle panel is the densitometry analysis of the blot, and the bottom panel the ethidium bromide stained 18S and 28S bands indicating equal loading of the lanes. This is a representative of three experiments.
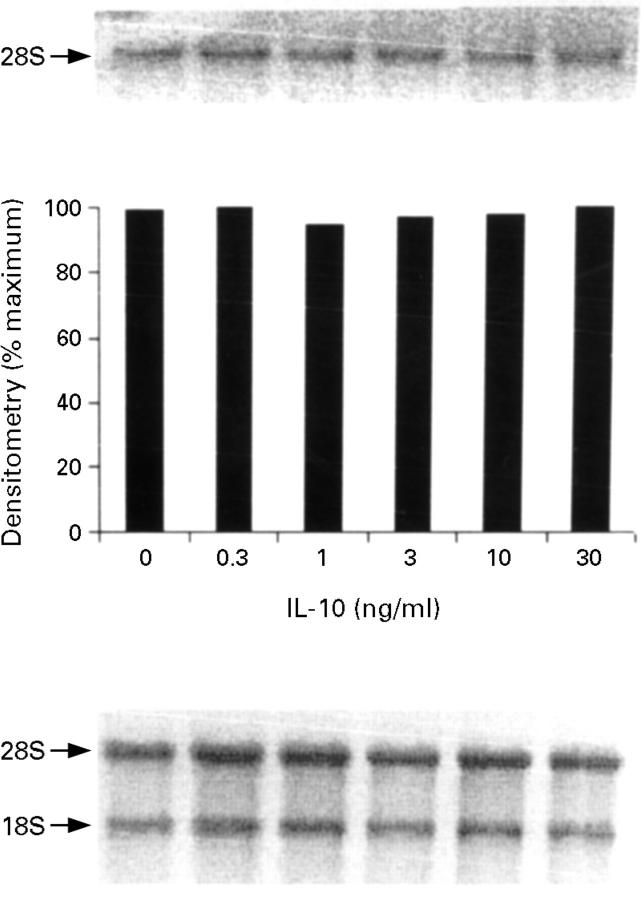
Figure 7 .
Inducible nitric oxide synthase (iNOS) mRNA expression by HT-29 cells after treatment with interleukin (IL)-1α (10 ng/ml)/interferon γ (IFN-γ) (300 U/ml)/tumour necrosis factor α (TNF-α) (100 ng/ml) in the presence of increasing concentrations of IL-10. The proinflammatory cytokines were added after one hour of pretreatment with IL-10 and incubated for 12 hours at 37°C. The top panel is the northern blot, the middle panel is the densitometry analysis of the blot, and the bottom panel the ethidium bromide stained 18S and 28S bands indicating equal loading of the lanes. This is a representative of three experiments.
Figure 8 .
Inducible nitric oxide synthase (iNOS) protein expression by HT-29 cells after stimulation with interleukin (IL)-1α (10 ng/ml)/interferon γ (IFN-γ) (300 U/ml)/tumour necrosis factor α (TNF-α) (100 ng/ml) in the presence of increasing concentrations of IL-13. HT-29 cells were pretreated for one hour with IL-13, then proinflammatory cytokines were added. HT-29 monolayers were scraped after 24 hours incubation at 37°C and total protein was extracted. iNOS protein expression was determined by western blot analysis, using an anti-human iNOS antibody. This is a representative of three experiments.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boughton-Smith N. K., Evans S. M., Hawkey C. J., Cole A. T., Balsitis M., Whittle B. J., Moncada S. Nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Lancet. 1993 Aug 7;342(8867):338–340. doi: 10.1016/0140-6736(93)91476-3. [DOI] [PubMed] [Google Scholar]
- Cenci E., Romani L., Mencacci A., Spaccapelo R., Schiaffella E., Puccetti P., Bistoni F. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur J Immunol. 1993 May;23(5):1034–1038. doi: 10.1002/eji.1830230508. [DOI] [PubMed] [Google Scholar]
- Doherty T. M., Kastelein R., Menon S., Andrade S., Coffman R. L. Modulation of murine macrophage function by IL-13. J Immunol. 1993 Dec 15;151(12):7151–7160. [PubMed] [Google Scholar]
- Doyle A. G., Herbein G., Montaner L. J., Minty A. J., Caput D., Ferrara P., Gordon S. Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-gamma. Eur J Immunol. 1994 Jun;24(6):1441–1445. doi: 10.1002/eji.1830240630. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchmann R., Schmitt E., Knolle P., Meyer zum Büschenfelde K. H., Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996 Apr;26(4):934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Miles A. M. Effects of aminosalicylates and immunosuppressive agents on nitric oxide-dependent N-nitrosation reactions. Biochem Pharmacol. 1994 May 18;47(10):1897–1902. doi: 10.1016/0006-2952(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Grisham M. B. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994 Sep 24;344(8926):859–861. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Ware K., Gilleland H. E., Jr, Gilleland L. B., Abell C. L., Yamada T. Neutrophil-mediated nitrosamine formation: role of nitric oxide in rats. Gastroenterology. 1992 Oct;103(4):1260–1266. doi: 10.1016/0016-5085(92)91513-4. [DOI] [PubMed] [Google Scholar]
- Hartley M. G., Hudson M. J., Swarbrick E. T., Hill M. J., Gent A. E., Hellier M. D., Grace R. H. The rectal mucosa-associated microflora in patients with ulcerative colitis. J Med Microbiol. 1992 Feb;36(2):96–103. doi: 10.1099/00222615-36-2-96. [DOI] [PubMed] [Google Scholar]
- Hutcheson I. R., Whittle B. J., Boughton-Smith N. K. Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol. 1990 Dec;101(4):815–820. doi: 10.1111/j.1476-5381.1990.tb14163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- Kasama T., Strieter R. M., Lukacs N. W., Burdick M. D., Kunkel S. L. Regulation of neutrophil-derived chemokine expression by IL-10. J Immunol. 1994 Apr 1;152(7):3559–3569. [PubMed] [Google Scholar]
- Kitagawa H., Takeda F., Kohei H. Effect of endothelium-derived relaxing factor on the gastric lesion induced by HCl in rats. J Pharmacol Exp Ther. 1990 Jun;253(3):1133–1137. [PubMed] [Google Scholar]
- Kolios G., Brown Z., Robson R. L., Robertson D. A., Westwick J. Inducible nitric oxide synthase activity and expression in a human colonic epithelial cell line, HT-29. Br J Pharmacol. 1995 Dec;116(7):2866–2872. doi: 10.1111/j.1476-5381.1995.tb15938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolios G., Robertson D. A., Jordan N. J., Minty A., Caput D., Ferrara P., Westwick J. Interleukin-8 production by the human colon epithelial cell line HT-29: modulation by interleukin-13. Br J Pharmacol. 1996 Sep;119(2):351–359. doi: 10.1111/j.1476-5381.1996.tb15993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzik T., Lügering N., Adolf M., Domschke W., Stoll R. Synergistic effect of immunoregulatory cytokines on peripheral blood monocytes from patients with inflammatory bowel disease. Dig Dis Sci. 1997 Apr;42(4):805–812. doi: 10.1023/a:1018872332387. [DOI] [PubMed] [Google Scholar]
- Kucharzik T., Lügering N., Weigelt H., Adolf M., Domschke W., Stoll R. Immunoregulatory properties of IL-13 in patients with inflammatory bowel disease; comparison with IL-4 and IL-10. Clin Exp Immunol. 1996 Jun;104(3):483–490. doi: 10.1046/j.1365-2249.1996.39750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzik T., Stoll R., Lügering N., Domschke W. Circulating antiinflammatory cytokine IL-10 in patients with inflammatory bowel disease (IBD). Clin Exp Immunol. 1995 Jun;100(3):452–456. doi: 10.1111/j.1365-2249.1995.tb03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon N. S., Stuehr D. J., Nathan C. F. Inhibition of tumor cell ribonucleotide reductase by macrophage-derived nitric oxide. J Exp Med. 1991 Oct 1;174(4):761–767. doi: 10.1084/jem.174.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichtman S. N., Sartor R. B. Examining the role of inflammatory cytokines in chronic inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1993 Apr;16(3):239–240. doi: 10.1097/00005176-199304000-00002. [DOI] [PubMed] [Google Scholar]
- Lundberg J. O., Hellström P. M., Lundberg J. M., Alving K. Greatly increased luminal nitric oxide in ulcerative colitis. Lancet. 1994 Dec 17;344(8938):1673–1674. doi: 10.1016/s0140-6736(94)90460-x. [DOI] [PubMed] [Google Scholar]
- MacNaughton W. K., Cirino G., Wallace J. L. Endothelium-derived relaxing factor (nitric oxide) has protective actions in the stomach. Life Sci. 1989;45(20):1869–1876. doi: 10.1016/0024-3205(89)90540-7. [DOI] [PubMed] [Google Scholar]
- MacNaughton W. K. Nitric oxide-donating compounds stimulate electrolyte transport in the guinea pig intestine in vitro. Life Sci. 1993;53(7):585–593. doi: 10.1016/0024-3205(93)90716-g. [DOI] [PubMed] [Google Scholar]
- Marfaing-Koka A., Devergne O., Gorgone G., Portier A., Schall T. J., Galanaud P., Emilie D. Regulation of the production of the RANTES chemokine by endothelial cells. Synergistic induction by IFN-gamma plus TNF-alpha and inhibition by IL-4 and IL-13. J Immunol. 1995 Feb 15;154(4):1870–1878. [PubMed] [Google Scholar]
- McKenzie A. N., Culpepper J. A., de Waal Malefyt R., Brière F., Punnonen J., Aversa G., Sato A., Dang W., Cocks B. G., Menon S. Interleukin 13, a T-cell-derived cytokine that regulates human monocyte and B-cell function. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3735–3739. doi: 10.1073/pnas.90.8.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton S. J., Shorthouse M., Hunter J. O. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993 Feb 20;341(8843):465–466. doi: 10.1016/0140-6736(93)90211-x. [DOI] [PubMed] [Google Scholar]
- Minty A., Chalon P., Derocq J. M., Dumont X., Guillemot J. C., Kaghad M., Labit C., Leplatois P., Liauzun P., Miloux B. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993 Mar 18;362(6417):248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- Misko T. P., Schilling R. J., Salvemini D., Moore W. M., Currie M. G. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993 Oct;214(1):11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moore K. W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T. R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Morris S. M., Jr, Billiar T. R. New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol. 1994 Jun;266(6 Pt 1):E829–E839. doi: 10.1152/ajpendo.1994.266.6.E829. [DOI] [PubMed] [Google Scholar]
- Nakane M., Schmidt H. H., Pollock J. S., Förstermann U., Murad F. Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett. 1993 Jan 25;316(2):175–180. doi: 10.1016/0014-5793(93)81210-q. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Niessner M., Volk B. A. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol. 1995 Sep;101(3):428–435. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussler A. K., Billiar T. R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993 Aug;54(2):171–178. [PubMed] [Google Scholar]
- Oswald I. P., Gazzinelli R. T., Sher A., James S. L. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992 Jun 1;148(11):3578–3582. [PubMed] [Google Scholar]
- Radford-Smith G., Jewell D. P. Cytokines and inflammatory bowel disease. Baillieres Clin Gastroenterol. 1996 Mar;10(1):151–164. doi: 10.1016/s0950-3528(96)90045-7. [DOI] [PubMed] [Google Scholar]
- Rennick D. M., Fort M. M., Davidson N. J. Studies with IL-10-/- mice: an overview. J Leukoc Biol. 1997 Apr;61(4):389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994 Feb;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Saura M., Martínez-Dalmau R., Minty A., Pérez-Sala D., Lamas S. Interleukin-13 inhibits inducible nitric oxide synthase expression in human mesangial cells. Biochem J. 1996 Jan 15;313(Pt 2):641–646. doi: 10.1042/bj3130641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Heinig T., Thiele H. G., Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995 May;108(5):1434–1444. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Scott S., Weidner J. R., Mumford R. A., Riehl T. E., Stenson W. F. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996 Oct;111(4):871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Tepperman B. L., Brown J. F., Korolkiewicz R., Whittle B. J. Nitric oxide synthase activity, viability and cyclic GMP levels in rat colonic epithelial cells: effect of endotoxin challenge. J Pharmacol Exp Ther. 1994 Dec;271(3):1477–1482. [PubMed] [Google Scholar]
- Uckun F. M., Schieven G. L., Tuel-Ahlgren L. M., Dibirdik I., Myers D. E., Ledbetter J. A., Song C. W. Tyrosine phosphorylation is a mandatory proximal step in radiation-induced activation of the protein kinase C signaling pathway in human B-lymphocyte precursors. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):252–256. doi: 10.1073/pnas.90.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., de Vries J. E. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994 Jan;15(1):19–26. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Figdor C. G., Huijbens R., Mohan-Peterson S., Bennett B., Culpepper J., Dang W., Zurawski G., de Vries J. E. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993 Dec 1;151(11):6370–6381. [PubMed] [Google Scholar]