Abstract
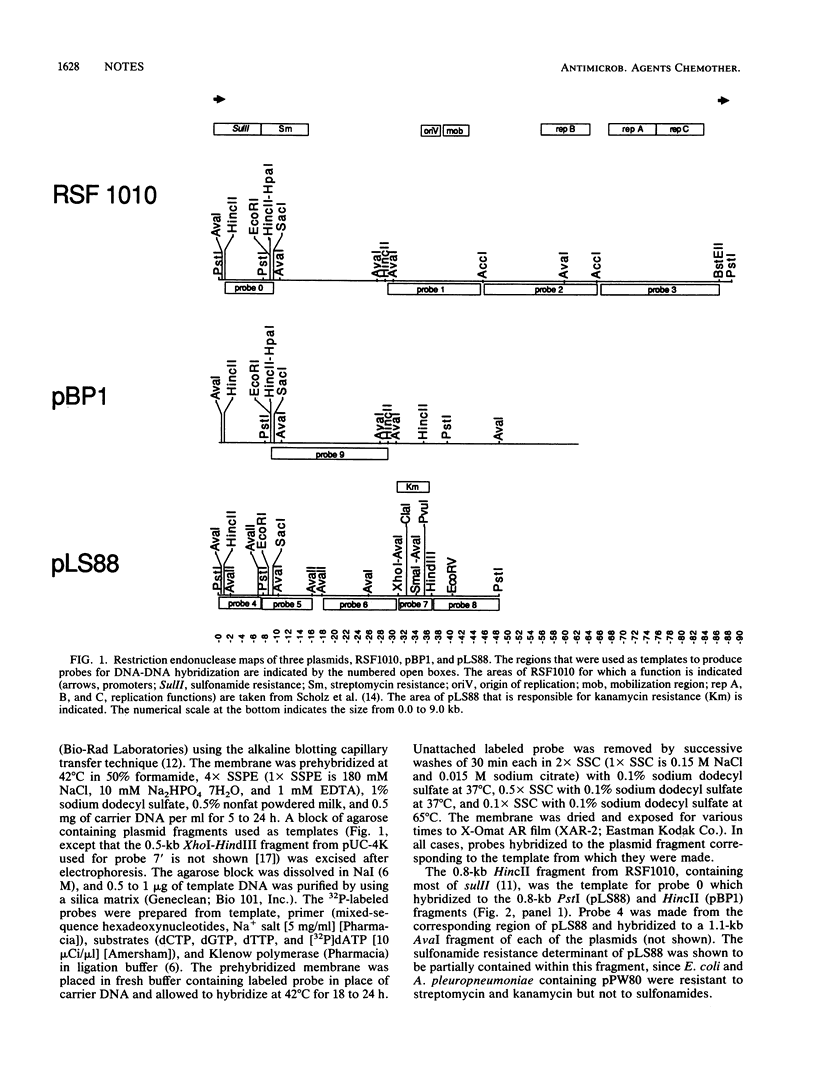
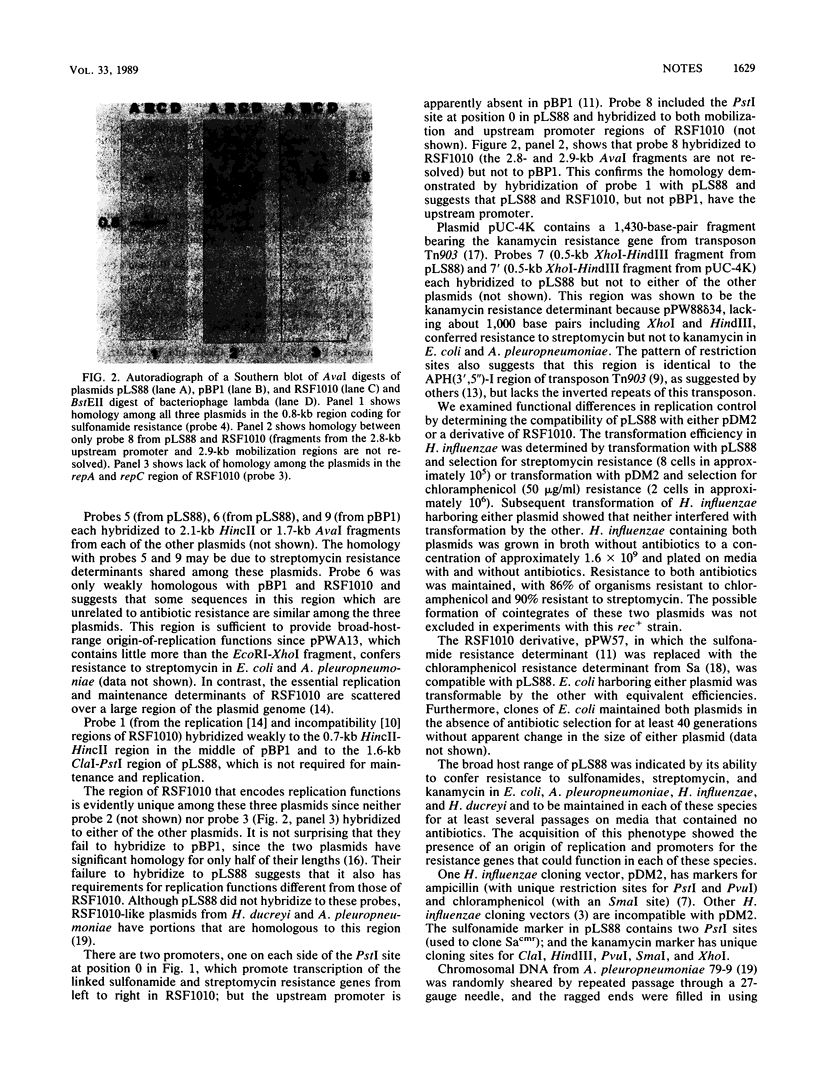
Plasmid pLS88 from a clinical isolate of Haemophilus ducreyi encoded resistance determinants for sulfonamides and streptomycin related to those of RSF1010 and for kanamycin related to Tn903 but lacked the inverted repeats of the transposon. Its host range included Haemophilus influenzae, Actinobacillus pleuropneumoniae, and Escherichia coli; and it was compatible with pDM2 and RSF1010.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L., Bendler J. W., Setlow J. K. Plasmid transformation in Haemophilus influenzae. J Bacteriol. 1981 Feb;145(2):1099–1101. doi: 10.1128/jb.145.2.1099-1101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton W. L., Brunton J. L., Slaney L., MacLean I. Plasmid-mediated sulfonamide resistance in Haemophilus ducreyi. Antimicrob Agents Chemother. 1982 Jan;21(1):159–165. doi: 10.1128/aac.21.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. B., Pifer M. L. Plasmid cloning vectors resistant to ampicillin and tetracycline which can replicate in both E. coli and Haemophilus cells. Gene. 1982 Apr;18(1):101–105. doi: 10.1016/0378-1119(82)90062-2. [DOI] [PubMed] [Google Scholar]
- Haring V., Scholz P., Scherzinger E., Frey J., Derbyshire K., Hatfull G., Willetts N. S., Bagdasarian M. Protein RepC is involved in copy number control of the broad host range plasmid RSF1010. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6090–6094. doi: 10.1073/pnas.82.18.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D., Clayton N. L., Setlow J. K. A plasmid cloning vehicle for Haemophilus influenzae and Escherichia coli. J Bacteriol. 1982 Sep;151(3):1605–1607. doi: 10.1128/jb.151.3.1605-1607.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Dower W. J., Tompkins L. S. High-voltage electroporation of bacteria: genetic transformation of Campylobacter jejuni with plasmid DNA. Proc Natl Acad Sci U S A. 1988 Feb;85(3):856–860. doi: 10.1073/pnas.85.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Sugisaki H., Takanami M. Nucleotide sequence of the kanamycin resistance transposon Tn903. J Mol Biol. 1981 Apr 5;147(2):217–226. doi: 10.1016/0022-2836(81)90438-1. [DOI] [PubMed] [Google Scholar]
- Persson C., Nordström K. Control of replication of the broad host range plasmid RSF1010: the incompatibility determinant consists of directly repeated DNA sequences. Mol Gen Genet. 1986 Apr;203(1):189–192. doi: 10.1007/BF00330402. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rådström P., Swedberg G. RSF1010 and a conjugative plasmid contain sulII, one of two known genes for plasmid-borne sulfonamide resistance dihydropteroate synthase. Antimicrob Agents Chemother. 1988 Nov;32(11):1684–1692. doi: 10.1128/aac.32.11.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson-le Pors M. J., Casin I. M., Collatz E. Plasmid-mediated aminoglycoside phosphotransferases in Haemophilus ducreyi. Antimicrob Agents Chemother. 1985 Aug;28(2):315–319. doi: 10.1128/aac.28.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz P., Haring V., Wittmann-Liebold B., Ashman K., Bagdasarian M., Scherzinger E. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene. 1989 Feb 20;75(2):271–288. doi: 10.1016/0378-1119(89)90273-4. [DOI] [PubMed] [Google Scholar]
- Taylor D. N., Echeverria P., Hanchalay S., Pitarangsi C., Slootmans L., Piot P. Antimicrobial susceptibility and characterization of outer membrane proteins of Haemophilus ducreyi isolated in Thailand. J Clin Microbiol. 1985 Mar;21(3):442–444. doi: 10.1128/jcm.21.3.442-444.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Grinsted J. Physical and genetic analysis of the Inc-W group plasmids R388, Sa, and R7K. Plasmid. 1982 May;7(3):239–250. doi: 10.1016/0147-619x(82)90005-1. [DOI] [PubMed] [Google Scholar]
- Willson P. J., Deneer H. G., Potter A., Albritton W. Characterization of a streptomycin-sulfonamide resistance plasmid from Actinobacillus pleuropneumoniae. Antimicrob Agents Chemother. 1989 Feb;33(2):235–238. doi: 10.1128/aac.33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Treeck U., Schmidt F., Wiedemann B. Molecular nature of a streptomycin and sulfonamide resistance plasmid (pBP1) prevalent in clinical Escherichia coli strains and integration of an ampicillin resistance transposon (TnA). Antimicrob Agents Chemother. 1981 Mar;19(3):371–380. doi: 10.1128/aac.19.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]



