Abstract
Background—The mechanisms underlying the suppression of somatostatin dependent reflexes in Helicobacter pylori infection are not fully determined. The H pylori product N -methylhistamine and inflammatory mediators such as tumour necrosis factor-α (TNF-α) may be responsible for the alterations in somatostatin release. Aims—To examine the effect of N -methylhistamine on somatostatin release from cultured somatostatin-secreting D-cells. Methods—Rabbit fundic D-cells were obtained by collagenase-EDTA digestion and enriched by centrifugal elutriation and cultured for 40 hours. The effects of N -methylhistamine on somatostatin release soon after stimulation (two hours) and after more prolonged exposure (24 hours) were assessed. Results—N -Methylhistamine (1 nM-1 µM) had no effect on basal or carbachol or adrenaline stimulated release over two hours. Similarly with prolonged exposure no effect on somatostatin cell content or release was identified. In contrast, TNF-α (24 hours) led to a dose dependent fall in both somatostatin content and release. Conclusions—N -Methylhistamine had no direct inhibitory effects on D-cells, but TNF-α both significantly reduced the cellular content and inhibited release. Inflammatory cytokines, rather than N -methylhistamine, are therefore likely to be responsible for directly inhibiting D-cell function in H pylori infection.
Keywords: D-cell; Helicobacter pylori; H3 receptor; N -methylhistamine; somatostatin; tumour necrosis factor α
Full Text
The Full Text of this article is available as a PDF (157.2 KB).
Figure 1 .
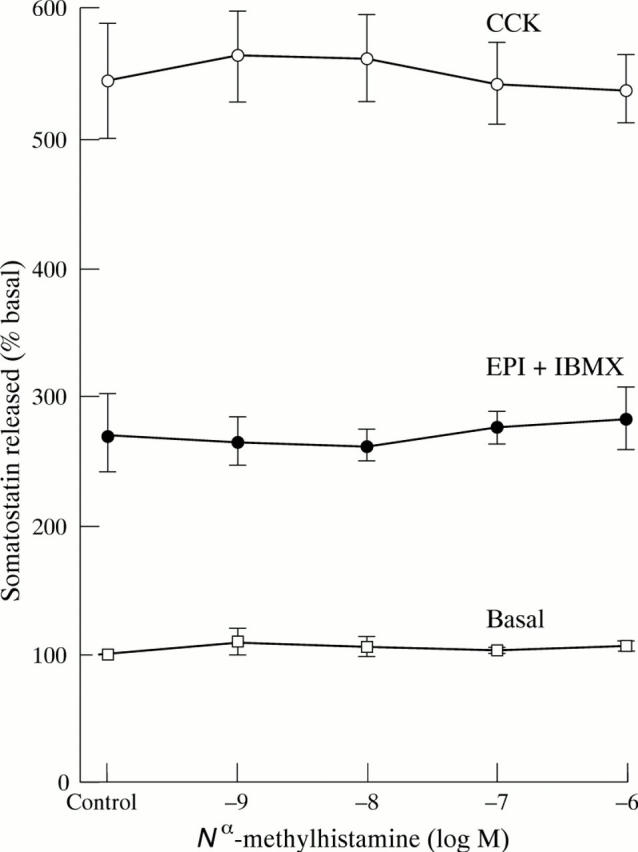
Effect of N -methylhistamine on somatostatin release from cultured D-cells. Somatostatin release over two hours in the presence of increasing concentrations of N -methylhistamine was measured in the basal state or when cells were co-stimulated with cholecystokinin 10 nM (CCK) or adrenaline (EPI) and isobutylmethyxanthine (IBMX) both 100 µM. Values are mean (SEM), n = 3.
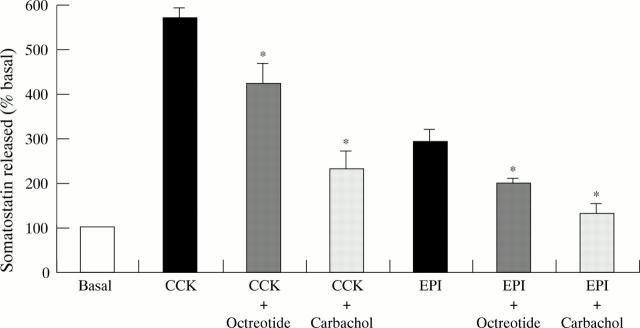
Figure 2 .
Effect of carbachol (10 µM) and octreotide (10 nM) on somatostatin release stimulated by cholecystokinin (CCK; 10 nM) and adrenaline with isobutylmethylxanthine (both 100 µM; EPI). Values are mean (SEM), n = 3. *p<0.05 compared with the stimulated somatostatin release.
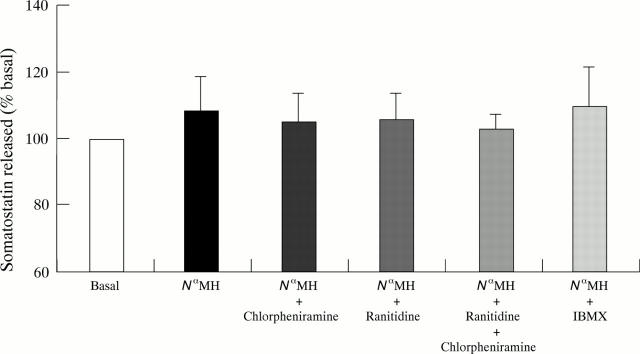
Figure 3 .
Effect of combining H1 receptor antagonism (chlorpheniramine 10 µM), H2 receptor antagonism (ranitidine 10 µM), and the phosphodiesterase inhibitor isobutylmethylxanthine (IBMX, 100 µM) on somatostatin release from D-cells stimulated with N -methylhistamine (100 nM) (N MH). Values are mean (SEM), n = 3.
Figure 4 .
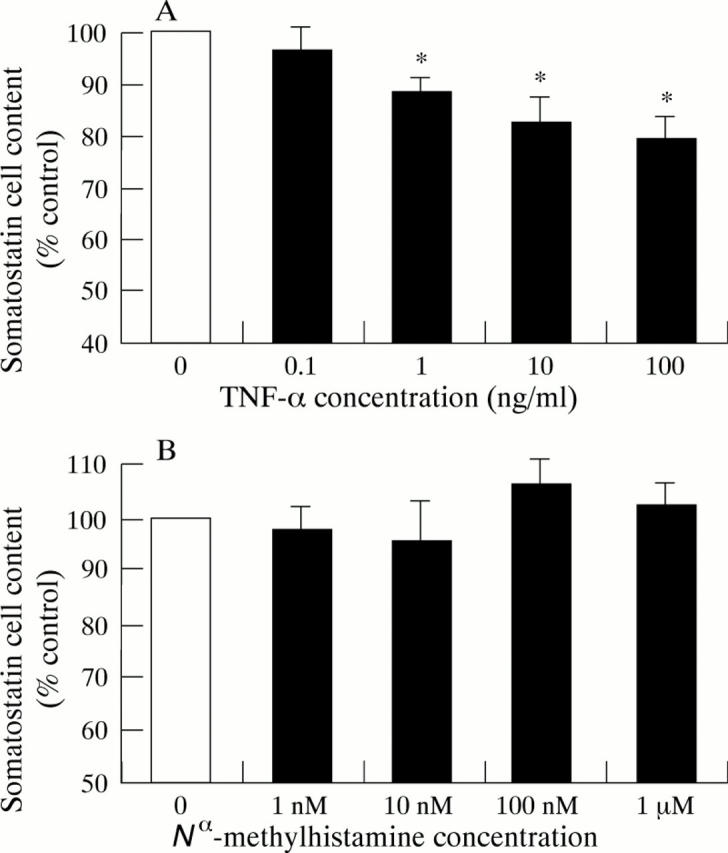
Effect of 24 hours preincubation with tumour necrosis factor-α (TNF-α) (A) and N -methylhistamine (B) on the somatostatin content of cultured D-cells. Results are expressed as percentage of control cells on the same culture plate. Values are mean (SEM), n = 5. *p<0.05 compared with control (no TNF-α).
Figure 5 .
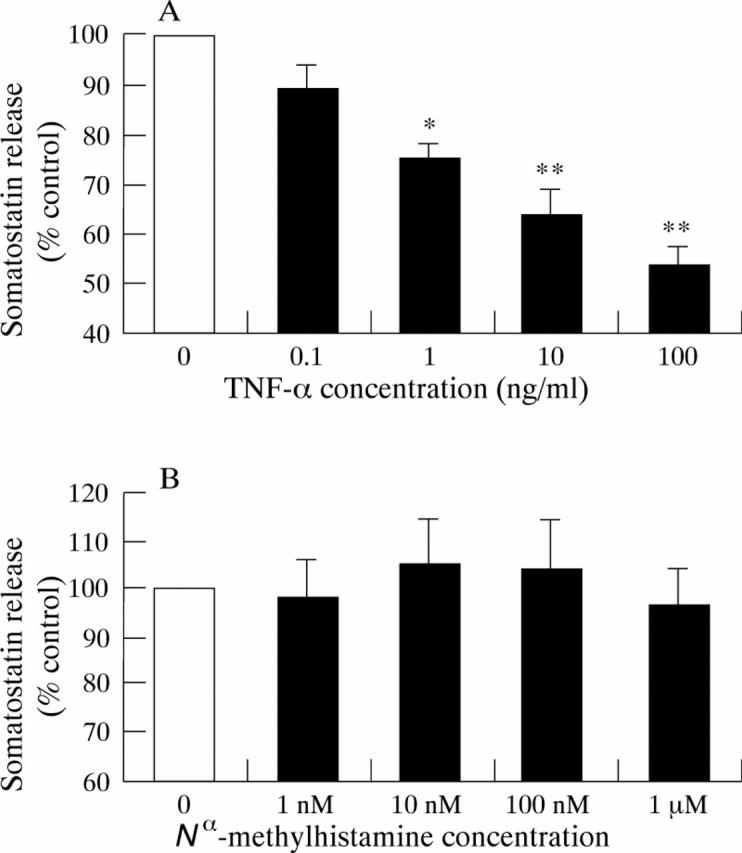
Effect of 24 hours preincubation with tumour necrosis factor (TNF-α) (A) and N -methylhistamine (B) on the somatostatin release from cultured D-cells in response to cholecystokinin 10 nM. Results are expressed as percentage of control cells on the same culture plate. Values are mean (SEM), n = 5. *p<0.05, **p<0.01 compared with controls (no TNF-α).
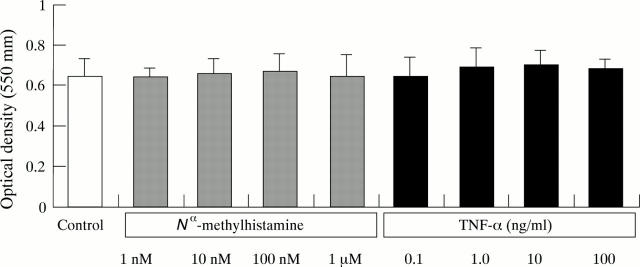
Figure 6 .
Effect of N -methylhistamine and tumour necrosis factor-α (TNF-α) on cell numbers and viability. After 24 hours incubation in test agents, viable cell numbers were measured by MTT assay. Cell numbers were quantified by measuring the optical density at 550 nm of the extracted formazan product. Values are mean (SEM), n = 3.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bado A., Hervatin F., Lewin M. J. Pharmacological evidence for histamine H3 receptor in the control of gastric acid secretion in cats. Am J Physiol. 1991 Apr;260(4 Pt 1):G631–G635. doi: 10.1152/ajpgi.1991.260.4.G631. [DOI] [PubMed] [Google Scholar]
- Beales I. L., Calam J. Effect of N alpha-methyl-histamine on acid secretion in isolated cultured rabbit parietal cells: implications for Helicobacter pylori associated gastritis and gastric physiology. Gut. 1997 Jan;40(1):14–19. doi: 10.1136/gut.40.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales I. L., Calam J. Helicobacter pylori infection and tumour necrosis factor-alpha increase gastrin release from human gastric antral fragments. Eur J Gastroenterol Hepatol. 1997 Aug;9(8):773–777. doi: 10.1097/00042737-199708000-00007. [DOI] [PubMed] [Google Scholar]
- Beales I. L., Calam J. Truncated glucagon-like peptide-1 and oxyntomodulin stimulate somatostatin release from rabbit fundic D-cells in primary culture. Exp Physiol. 1996 Nov;81(6):1039–1041. doi: 10.1113/expphysiol.1996.sp003988. [DOI] [PubMed] [Google Scholar]
- Beales I. L., Post L., Calam J., Yamada T., Delvalle J. Tumour necrosis factor alpha stimulates gastrin release from canine and human antral G cells: possible mechanism of the Helicobacter pylori-gastrin link. Eur J Clin Invest. 1996 Jul;26(7):609–611. doi: 10.1046/j.1365-2362.1996.2040517.x. [DOI] [PubMed] [Google Scholar]
- Beales I., Calam J., Post L., Srinivasan S., Yamada T., DelValle J. Effect of transforming growth factor alpha and interleukin 8 on somatostatin release from canine fundic D cells. Gastroenterology. 1997 Jan;112(1):136–143. doi: 10.1016/s0016-5085(97)70228-2. [DOI] [PubMed] [Google Scholar]
- Bertaccini G., Coruzzi G. An update on histamine H3 receptors and gastrointestinal functions. Dig Dis Sci. 1995 Sep;40(9):2052–2063. doi: 10.1007/BF02208678. [DOI] [PubMed] [Google Scholar]
- Bertaccini G., Coruzzi G., Poli E. Review article: the histamine H3-receptor: a novel prejunctional receptor regulating gastrointestinal function. Aliment Pharmacol Ther. 1991 Dec;5(6):585–591. doi: 10.1111/j.1365-2036.1991.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Buchan A. M., Curtis S. B., Meloche R. M. Release of somatostatin immunoreactivity from human antral D cells in culture. Gastroenterology. 1990 Sep;99(3):690–696. doi: 10.1016/0016-5085(90)90956-2. [DOI] [PubMed] [Google Scholar]
- Coruzzi G., Adami M., Bertaccini G. Histamine H3 receptors are not involved in the regulation of rat gastric secretion. Pharmacology. 1992;44(4):190–195. doi: 10.1159/000138918. [DOI] [PubMed] [Google Scholar]
- Coruzzi G., Bertaccini G., Schwartz J. C. Evidence that histamine H3 receptors are involved in the control of gastric acid secretion in the conscious cat. Naunyn Schmiedebergs Arch Pharmacol. 1991 Feb;343(2):225–227. doi: 10.1007/BF00168615. [DOI] [PubMed] [Google Scholar]
- Courillon-Mallet A., Launay J. M., Roucayrol A. M., Callebert J., Emond J. P., Tabuteau F., Cattan D. Helicobacter pylori infection: physiopathologic implication of N alpha-methyl histamine. Gastroenterology. 1995 Apr;108(4):959–966. doi: 10.1016/0016-5085(95)90190-6. [DOI] [PubMed] [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986 May 22;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Dial E. J., Hall L. R., Romero J. J., Lichtenberger L. M. Rats with gastritis have increased sensitivity to the gastrin stimulatory effects of luminal ammonia. Gastroenterology. 1996 Mar;110(3):801–808. doi: 10.1053/gast.1996.v110.pm8608890. [DOI] [PubMed] [Google Scholar]
- Gibbons A. H., Legon S., Walker M. M., Ghatei M., Calam J. The effect of gastrin-releasing peptide on gastrin and somatostatin messenger RNAs in humans infected with Helicobacter pylori. Gastroenterology. 1997 Jun;112(6):1940–1947. doi: 10.1053/gast.1997.v112.pm9178686. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Go M. F., Lew G. M., Genta R. M., Rehfeld J. F. Helicobacter pylori infection and exaggerated gastrin release. Effects of inflammation and progastrin processing. Scand J Gastroenterol. 1993 Aug;28(8):690–694. doi: 10.3109/00365529309098274. [DOI] [PubMed] [Google Scholar]
- Greenberg G. R., Fung L., Pokol-Daniel S. Regulation of somatostatin-14 and -28 secretion by gastric acid in dogs: differential role of cholecystokinin. Gastroenterology. 1993 Nov;105(5):1387–1395. doi: 10.1016/0016-5085(93)90143-z. [DOI] [PubMed] [Google Scholar]
- Jones T. H., Kennedy R. L. Cytokines and hypothalamic-pituitary function. Cytokine. 1993 Nov;5(6):531–538. doi: 10.1016/s1043-4666(05)80001-8. [DOI] [PubMed] [Google Scholar]
- Kaneko H., Nakada K., Mitsuma T., Uchida K., Furusawa A., Maeda Y., Morise K. Helicobacter pylori infection induces a decrease in immunoreactive-somatostatin concentrations of human stomach. Dig Dis Sci. 1992 Mar;37(3):409–416. doi: 10.1007/BF01307736. [DOI] [PubMed] [Google Scholar]
- Korte A., Myers J., Shih N. Y., Egan R. W., Clark M. A. Characterization and tissue distribution of H3 histamine receptors in guinea pigs by N alpha-methylhistamine. Biochem Biophys Res Commun. 1990 May 16;168(3):979–986. doi: 10.1016/0006-291x(90)91125-c. [DOI] [PubMed] [Google Scholar]
- Ligumsky M., Wengrower D., Karmeli F., Rachmilewitz D. Somatostatin release by human gastric mucosa. Studies in peptic ulcer disease and pernicious anemia. Scand J Gastroenterol. 1988 Aug;23(6):687–690. doi: 10.3109/00365528809093933. [DOI] [PubMed] [Google Scholar]
- Manela F. D., Ren J., Gao J., McGuigan J. E., Harty R. F. Calcitonin gene-related peptide modulates acid-mediated regulation of somatostatin and gastrin release from rat antrum. Gastroenterology. 1995 Sep;109(3):701–706. doi: 10.1016/0016-5085(95)90376-3. [DOI] [PubMed] [Google Scholar]
- Moss S. F., Legon S., Bishop A. E., Polak J. M., Calam J. Effect of Helicobacter pylori on gastric somatostatin in duodenal ulcer disease. Lancet. 1992 Oct 17;340(8825):930–932. doi: 10.1016/0140-6736(92)92816-x. [DOI] [PubMed] [Google Scholar]
- Olbe L., Hamlet A., Dalenbäck J., Fändriks L. A mechanism by which Helicobacter pylori infection of the antrum contributes to the development of duodenal ulcer. Gastroenterology. 1996 May;110(5):1386–1394. doi: 10.1053/gast.1996.v110.pm8613042. [DOI] [PubMed] [Google Scholar]
- Park J., Chiba T., Yokotani K., DelValle J., Yamada T. Somatostatin receptors on canine fundic D-cells: evidence for autocrine regulation of gastric somatostatin. Am J Physiol. 1989 Aug;257(2 Pt 1):G235–G241. doi: 10.1152/ajpgi.1989.257.2.G235. [DOI] [PubMed] [Google Scholar]
- Peek R. M., Jr, Blaser M. J. Pathophysiology of Helicobacter pylori-induced gastritis and peptic ulcer disease. Am J Med. 1997 Feb;102(2):200–207. doi: 10.1016/s0002-9343(96)00273-2. [DOI] [PubMed] [Google Scholar]
- Prinz C., Kajimura M., Scott D. R., Mercier F., Helander H. F., Sachs G. Histamine secretion from rat enterochromaffinlike cells. Gastroenterology. 1993 Aug;105(2):449–461. doi: 10.1016/0016-5085(93)90719-s. [DOI] [PubMed] [Google Scholar]
- Schubert M. L., Edwards N. F., Makhlouf G. M. Regulation of gastric somatostatin secretion in the mouse by luminal acidity: a local feedback mechanism. Gastroenterology. 1988 Feb;94(2):317–322. doi: 10.1016/0016-5085(88)90418-0. [DOI] [PubMed] [Google Scholar]
- Soldani G., Intorre L., Bertini S., Luchetti E., Coruzzi G., Bertaccini G. Regulation of gastric acid secretion by histamine H3 receptors in the dog: an investigation into the site of action. Naunyn Schmiedebergs Arch Pharmacol. 1994 Aug;350(2):218–223. doi: 10.1007/BF00241100. [DOI] [PubMed] [Google Scholar]
- Soldani G., Mengozzi G., Intorre L., De Giorgi G., Coruzzi G., Bertaccini G. Histamine H3 receptor-mediated inhibition of gastric acid secretion in conscious dogs. Naunyn Schmiedebergs Arch Pharmacol. 1993 Jan;347(1):61–65. doi: 10.1007/BF00168773. [DOI] [PubMed] [Google Scholar]
- Vuyyuru L., Harrington L., Arimura A., Schubert M. L. Reciprocal inhibitory paracrine pathways link histamine and somatostatin secretion in the fundus of the stomach. Am J Physiol. 1997 Jul;273(1 Pt 1):G106–G111. doi: 10.1152/ajpgi.1997.273.1.G106. [DOI] [PubMed] [Google Scholar]
- Vuyyuru L., Schubert M. L., Harrington L., Arimura A., Makhlouf G. M. Dual inhibitory pathways link antral somatostatin and histamine secretion in human, dog, and rat stomach. Gastroenterology. 1995 Nov;109(5):1566–1574. doi: 10.1016/0016-5085(95)90645-2. [DOI] [PubMed] [Google Scholar]
- Vuyyuru L., Schubert M. L. Histamine, acting via H3 receptors, inhibits somatostatin and stimulates acid secretion in isolated mouse stomach. Gastroenterology. 1997 Nov;113(5):1545–1552. doi: 10.1053/gast.1997.v113.pm9352856. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Kubota Y., Sawada T., Muto T. Distribution and quantification of somatostatin in inflammatory disease. Dis Colon Rectum. 1992 May;35(5):488–494. doi: 10.1007/BF02049408. [DOI] [PubMed] [Google Scholar]
- Yamada T., Soll A. H., Park J., Elashoff J. Autonomic regulation of somatostatin release: studies with primary cultures of canine fundic mucosal cells. Am J Physiol. 1984 Nov;247(5 Pt 1):G567–G573. doi: 10.1152/ajpgi.1984.247.5.G567. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Mochizuki T., Okakura-Mochizuki K., Uno A., Yamatodani A. Thioperamide, a histamine H3 receptor antagonist, increases GABA release from the rat hypothalamus. Methods Find Exp Clin Pharmacol. 1997 Jun;19(5):289–298. [PubMed] [Google Scholar]