Abstract
Background—K88 positive enterotoxigenic Escherichia coli (K88+ ETEC) is an important cause of diarrhoea in young piglets. K88+ ETEC pathogenesis relies on attachment to specific glycoprotein receptors located on the intestinal mucosa. Proteolytic treatment of these receptors in vitro and in vivo prevents attachment of K88+ ETEC to piglet small intestines and may be of clinical use to prevent K88+ ETEC pathogenesis. Aims—To determine whether bromelain, a proteolytic extract obtained from pineapple stems, would protect piglets against K88+ ETEC diarrhoea and to confirm and extend earlier findings on the effects of bromelain on K88+ ETEC receptors in vivo. Methods—Bromelain (0, 12.5, or 125 mg) was orally administered to just weaned piglets for 10 days. One day following commencement of bromelain treatment, piglets were challenged with K88+ ETEC (5 × 1010 K88ac:0149) for seven days. Intestinal contents from unchallenged piglets were obtained via an intestinal fistula, and tested for their ability to bind K88+ ETEC before and after bromelain treatment. Results—Both doses of bromelain were successful in reducing the incidence of K88+ ETEC diarrhoea and protected piglets from life threatening disease. Bromelain treated pigs also had significantly increased weight gain compared with untreated pigs. Bromelain only temporarily inhibited K88+ ETEC receptor activity, with receptor activity being regenerated 30 hours following treatment, consistent with the regeneration of new enterocytes. Conclusion—Results show that bromelain can temporarily inactivate ETEC receptors in vivo and protect against ETEC induced diarrhoea. Bromelain may therefore be an effective prophylaxis against ETEC infection.
Keywords: enterotoxigenic Escherichia coli; K88 ETEC; ETEC receptors; diarrhoea; bromelain
Full Text
The Full Text of this article is available as a PDF (124.9 KB).
Figure 1 .
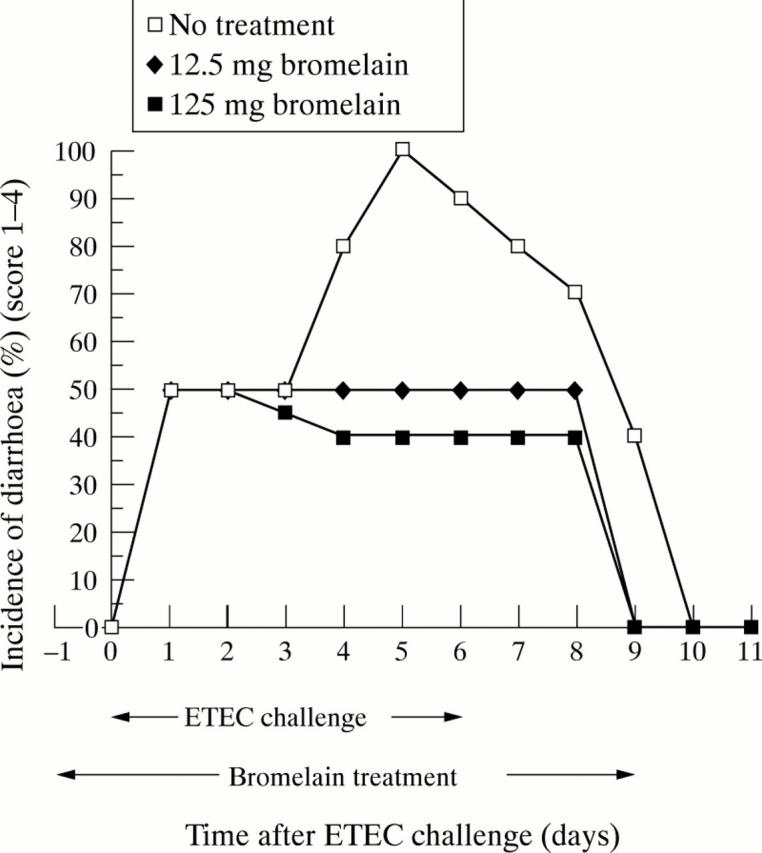
Incidence of diarrhoea (combined score 1 to 4) in piglets challenged with K88+ ETEC.
Figure 2 .

Effect of bromelain treatment on piglets challenged with K88+ ETEC. (A) Mean total disease score. (B) Mean number of life threatening disease scores.*p<0.05.
Figure 3 .
Effect of bromelain treatment on weight gain of piglets challenged with K88+ ETEC. *p<0.05;**p<0.01.
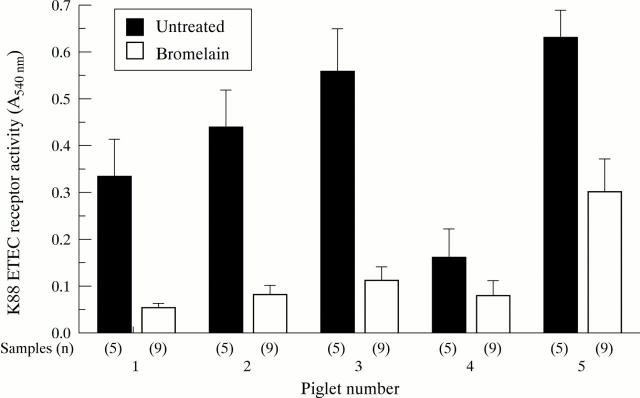
Figure 4 .
K88+ ETEC receptor activity of small intestinal contents of piglets before and after bromelain treatment. Bars represent the mean (SD) EIA activity (A540 nm) of intestinal samples taken from piglets before (n=5) and after (n=9) bromelain treatment.
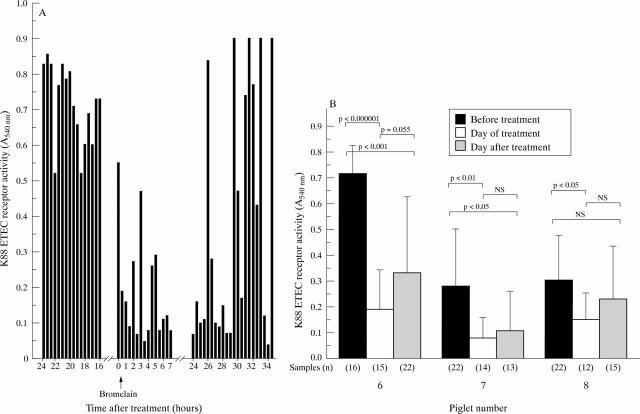
Figure 5 .
Change in K88+ ETEC receptor activity of small intestinal contents following bromelain treatment. (A) Results obtained from one piglet (no 6); receptor activity assayed at 30 minute intervals. (B) Mean receptor activity of intestinal contents for each day of sample collection. Numbers in parentheses indicate number of samples assayed. Significance determined by Student's t test for paired observations.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler D. S., Chandler H. M., Luke R. K., Tzipori S. R., Craven J. A. Screening of pig intestines for K88 non-adhesive phenotype by enzyme immunoassay. Vet Microbiol. 1986 Feb;11(1-2):153–161. doi: 10.1016/0378-1135(86)90015-5. [DOI] [PubMed] [Google Scholar]
- Chandler D. S., Mynott T. L., Luke R. K., Craven J. A. The distribution and stability of Escherichia coli K88 receptor in the gastrointestinal tract of the pig. Vet Microbiol. 1994 Jan;38(3):203–215. doi: 10.1016/0378-1135(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Dean E. A., Whipp S. C., Moon H. W. Age-specific colonization of porcine intestinal epithelium by 987P-piliated enterotoxigenic Escherichia coli. Infect Immun. 1989 Jan;57(1):82–87. doi: 10.1128/iai.57.1.82-87.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson A. K., Baker D. R., Bosworth B. T., Casey T. A., Benfield D. A., Francis D. H. Characterization of porcine intestinal receptors for the K88ac fimbrial adhesin of Escherichia coli as mucin-type sialoglycoproteins. Infect Immun. 1994 Dec;62(12):5404–5410. doi: 10.1128/iai.62.12.5404-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouricout M. A., Julien R. A. Pilus-mediated binding of bovine enterotoxigenic Escherichia coli to calf small intestinal mucins. Infect Immun. 1987 May;55(5):1216–1223. doi: 10.1128/iai.55.5.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynott T. L., Chandler D. S., Luke R. K. Efficacy of enteric-coated protease in preventing attachment of enterotoxigenic Escherichia coli and diarrheal disease in the RITARD model. Infect Immun. 1991 Oct;59(10):3708–3714. doi: 10.1128/iai.59.10.3708-3714.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynott T. L., Guandalini S., Raimondi F., Fasano A. Bromelain prevents secretion caused by Vibrio cholerae and Escherichia coli enterotoxins in rabbit ileum in vitro. Gastroenterology. 1997 Jul;113(1):175–184. doi: 10.1016/s0016-5085(97)70093-3. [DOI] [PubMed] [Google Scholar]
- Mynott T. L., Luke R. K., Chandler D. S. Detection of attachment of enterotoxigenic Escherichia coli (ETEC) to human small intestinal cells by enzyme immunoassay. FEMS Immunol Med Microbiol. 1995 Feb;10(3-4):207–218. doi: 10.1111/j.1574-695X.1995.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Mynott T. L., Luke R. K., Chandler D. S. Oral administration of protease inhibits enterotoxigenic Escherichia coli receptor activity in piglet small intestine. Gut. 1996 Jan;38(1):28–32. doi: 10.1136/gut.38.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J. M., Burrows M. R., Sellwood R., Gibbons R. A. A genetic basis for resistance to enteric disease caused by E. coli. Nature. 1975 Sep 11;257(5522):135–136. doi: 10.1038/257135a0. [DOI] [PubMed] [Google Scholar]
- Sellwood R., Gibbons R. A., Jones G. W., Rutter J. M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975 Aug;8(3):405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- Staley T. E., Wilson I. B. Soluble pig intestinal cell membrane components with affinities for E. coli K88+ antigen. Mol Cell Biochem. 1983;52(2):177–189. doi: 10.1007/BF00224926. [DOI] [PubMed] [Google Scholar]