Abstract
Aim—To determine the kinetics of platelet activating factor (PAF) and prostaglandin E2 (PGE2) receptor desensitisation during intestinal inflammation induced by trinitrobenzenesulphonic acid (TNB) instillation and to study the relation between receptor regulation, inflammatory lesions, and PAF content of the gut wall. Methods—Receptor desensitisation was assessed on isolated smooth muscle cells from the circular layer. PAF content of the intestinal wall was determined by thin layer chromatography and radioimmunoassay. Results—After an acute inflammatory phase on day 1, subacute changes appeared in TNB instilled ileum, with a maximal intensity on day 6. In control animals, PAF 10 nM and PGE2 10 nM provoked a maximal contraction in the range of 24% of cell shortening. On days 1 and 3 after intestinal instillation of TNB, PAF induced contraction was not altered whereas the effect of PGE2 was progressively desensitised (2 logM rightward shift of its concentration-response curve: Cmax = 1 µM; p<0.01). Between days 4 and 6, the concentration-response curve of PGE2 shifted by only 1 logM (p<0.05) whereas the curve of PAF induced contraction shifted by 2 logM (Cmax = 1 µM; p<0.01). The PAF content of the ileal wall was maximal between days 3 and 5 (300 ng/mg tissue). On days 10 and 15, PAF and PGE2 induced contractions were similar to those observed on day 1, and PAF content returned to basal. Conclusion—Inflammation induced by TNB instillation triggers PAF and PGE2 receptor desensitisation; this is dependent on the duration of inflammation and correlates with PAF content in the ileum. This receptor desensitisation may play a protective role by preventing overstimulation of intestinal smooth muscle cells.
Keywords: platelet activating factor receptor; prostaglandin E2 receptor; receptor desensitisation; intestinal inflammation; trinitrobenzensulphonic acid; smooth muscle cells
Full Text
The Full Text of this article is available as a PDF (224.3 KB).
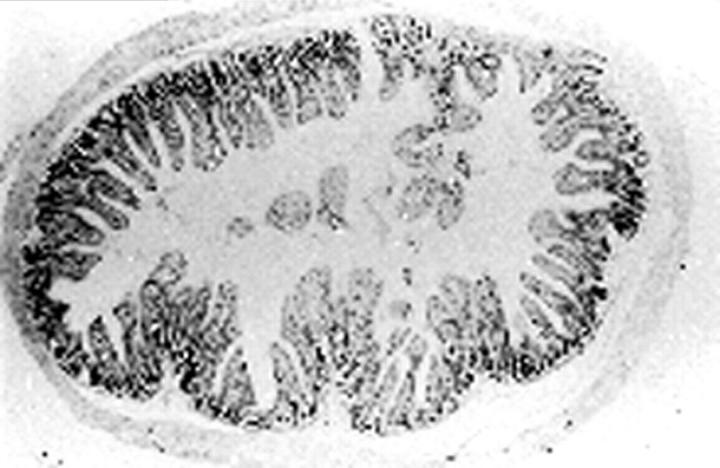
Figure 1 .
Normal histological pattern of the ileum six days after instillation of saline into the lumen (haemalun/eosin stain; original magnification × 6.3).
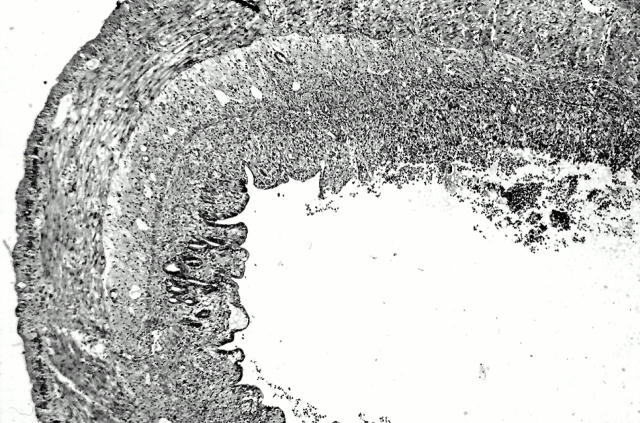
Figure 2 .
Acute inflammation induced by trinitrobenzenesulphonic acid (TNB) instillation into the lumen of guinea pig ileum. On day 1 after the instillation, the lesions are mainly characterised by the presence of oedema in the submucosa and the infiltration of mononuclear cells and neutrophils and mucosal erosions (haemalun/eosin stain; original magnification × 16).
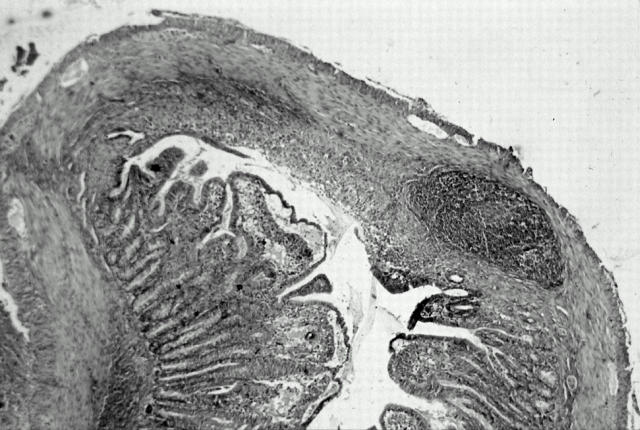
Figure 3 .
Second phase of the inflammation provoked by trinitrobenzenesulphonic acid (TNB) in guinea pig ileum. Villi are destroyed. The mucosa is largely eroded by deep ulcerations. Infiltration into the submucosa is maximal (day 3) (haemalun/eosin stain; original magnification × 6.3).
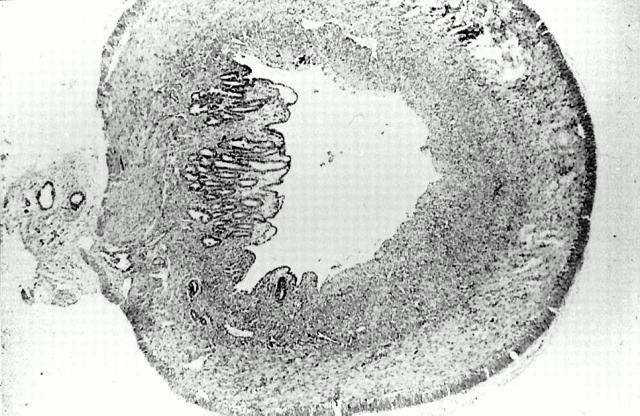
Figure 4 .
Subacute phase of inflammation with fibrosis in the submucosa. Ulcerations are less deep than in the earlier stages of inflammation (day 6) (haemalun/eosin stain; original magnification × 6.3).
Figure 5 .
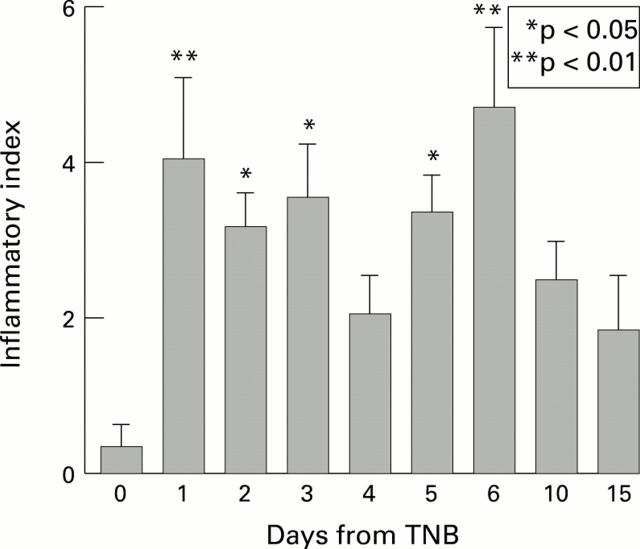
Inflammatory index characterising morphological changes in intestinal wall after instillation of trinitrobenzenesulphonic acid (TNB). Five animals were killed at each time point and the inflammatory index was measured for each of the samples. Data are shown as mean (SEM). The inflammatory index is statistically significantly different from that on day 0, except on days 4, 10, and 15.
Figure 6 .
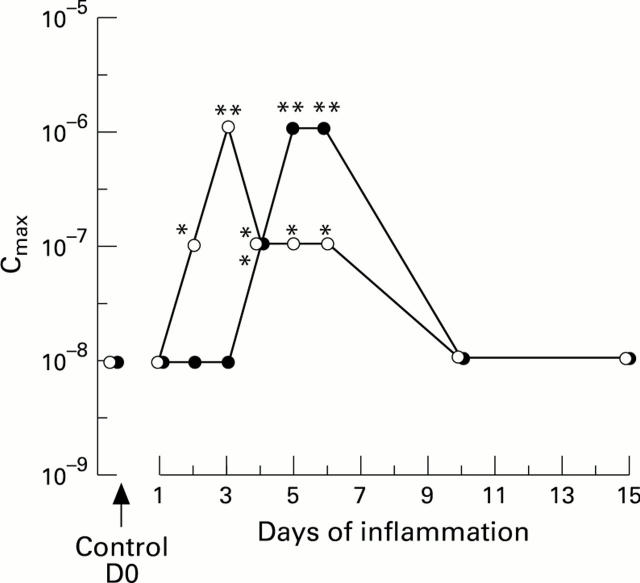
Kinetics of desensitisation of platelet activating factor (PAF; solid circles) and prostaglandin E2 (PGE2; open circles) receptors of smooth muscle cells of the circular layer, after intraluminal instillation of trinitrobenzenesulphonic acid (TNB) into guinea pig ileum (mean (SEM) of samples obtained at each time point from five different animals). The concentration of PAF and PGE2 inducing maximal contraction (Cmax) of the cells is expressed in logM and plotted against time elapsed between TNB instillation and the killing of the animals. Values of Cmax significantly different from control animals treated with saline: *p<0.05; **p<0.01.
Figure 7 .
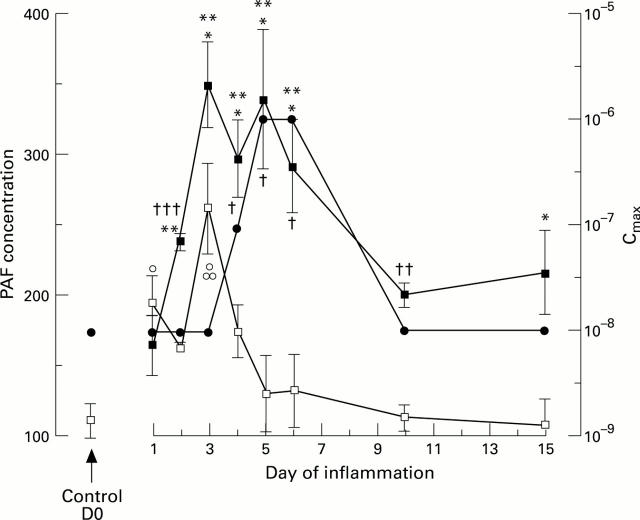
Time relationship of platelet activating factor (PAF) receptor desensitisation in intestinal smooth muscle cells (solid circle) after trinitrobenzenesulphonic acid (TNB) instillation and PAF content in the ileum, after intraluminal instillation of saline (open squares) or TNB (solid squares) in guinea pig ileum. PAF content was evaluated by thin layer chromatography on the various days after TNB treatment (mean (SEM) for samples obtained at each time from five different animals). The concentration of PAF inducing a maximal cell contraction did not change over time in saline treated animals and is not shown. Statistically significant differences are quoted as follows: *p<0.05, **p<0.01, ***p<0.001 for comparison between TNB treated animals and values at D0; °p<0.05, °°°p<0.001 for comparison between saline and values at D0; †p<0.05, ††p<0.01, †††p<0.001 for comparison between TNB and saline treated animals at the same time interval from surgery. On day 1 after intraluminal instillation of TNB, we observed a moderate increase in PAF content of the gut wall (165 (22) ng/mg tissue) (NS) (table 3). Three days after TNB treatment, PAF content was maximal in injured ileum (348 (31) ng/mg tissue) (p<0.001), and was significantly higher than that measured on day 3 in ileum from saline treated animals (p<0.01). The PAF content remained at this high level for the next 2 days (days 4 and 5). From day 6, it decreased and returned to basal level by day 10 (200 (9) ng/mg tissue). On day 15, the amount of PAF in the ileum from TNB treated animals increased again (215 (30) ng/mg tissue) and was significantly higher than that measured in untreated animals (day 0) (109 (13) ng/mg tissue) (p<0.05).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Capasso F., Tavares I. A., Bennett A. Release of platelet-activating factor (PAF) from human colon mucosa and its inhibition by 5-aminosalicylic acid. Drugs Exp Clin Res. 1991;17(7):351–353. [PubMed] [Google Scholar]
- Chao W., Liu H., Hanahan D. J., Olson M. S. Regulation of platelet-activating factor receptors in rat Kupffer cells. J Biol Chem. 1989 Dec 5;264(34):20448–20457. [PubMed] [Google Scholar]
- Chesney C. M., Pifer D. D., Huch K. M. Desensitization of human platelets by platelet activating factor. Biochem Biophys Res Commun. 1985 Feb 28;127(1):24–30. doi: 10.1016/s0006-291x(85)80120-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. D., Kao H. W., Tan S. T., Lechago J., Snape W. J., Jr Effect of acute experimental colitis on rabbit colonic smooth muscle. Am J Physiol. 1986 Oct;251(4 Pt 1):G538–G545. doi: 10.1152/ajpgi.1986.251.4.G538. [DOI] [PubMed] [Google Scholar]
- Denizot Y., Chaussade S., Colombel J. F., Benveniste J., Couturier D. Présence du médiateur de l'inflammation paf-acéther dans les selles de patients atteints d'entérocolite inflammatoire. C R Acad Sci III. 1991;312(7):329–333. [PubMed] [Google Scholar]
- Denizot Y., Chaussade S., Nathan N., Colombel J. F., Bossant M. J., Cherouki N., Benveniste J., Couturier D. PAF-acether and acetylhydrolase in stool of patients with Crohn's disease. Dig Dis Sci. 1992 Mar;37(3):432–437. doi: 10.1007/BF01307739. [DOI] [PubMed] [Google Scholar]
- Eliakim R., Karmeli F., Okon E., Rachmilewitz D. Octreotide effectively decreases mucosal damage in experimental colitis. Gut. 1993 Feb;34(2):264–269. doi: 10.1136/gut.34.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino H., Sugiyama S., Ohara A., Goto H., Tsukamoto Y., Ozawa T. Mechanism and prevention of chronic colonic inflammation with trinitrobenzene sulfonic acid in rats. Clin Exp Pharmacol Physiol. 1992 Oct;19(10):717–722. doi: 10.1111/j.1440-1681.1992.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Jeanneton O., Delvaux M., Botella A., Frexinos J., Bueno L. Homologous desensitization of PAF receptors via a PGE2-dependent pathway on intestinal smooth muscle. J Lipid Mediat Cell Signal. 1994 Sep;10(3):331–344. [PubMed] [Google Scholar]
- Jeanneton O., Delvaux M., Botella A., Frexinos J., Bueno L. Platelet-activating factor (PAF) induces a contraction of isolated smooth muscle cells from guinea pig ileum: intracellular pathway involved. J Pharmacol Exp Ther. 1993 Oct;267(1):31–37. [PubMed] [Google Scholar]
- Jeanneton O., Delvaux M., Frexinos J., Bueno L. Desensitization of platelet-activating factor receptors, induced by inflammation in guinea pig ileal smooth muscle cells. Gastroenterology. 1995 Jun;108(6):1666–1675. doi: 10.1016/0016-5085(95)90127-2. [DOI] [PubMed] [Google Scholar]
- Kald B., Olaison G., Sjödahl R., Tagesson C. Novel aspect of Crohn's disease: increased content of platelet-activating factor in ileal and colonic mucosa. Digestion. 1990;46(4):199–204. doi: 10.1159/000200346. [DOI] [PubMed] [Google Scholar]
- Keraly C. L., Benveniste J. Specific desensitization of rabbit platelets by platelet-activating factor (PAF-acether) and derivatives. Br J Haematol. 1982 Jun;51(2):313–322. [PubMed] [Google Scholar]
- Longo W. E., Polities G., Vernava A. M., 3rd, Deshpande Y., Niehoff M., Chandel B., Kulkarni A., Kaminski D. L. Platelet-activating factor mediates trinitrobenzene induced colitis. Prostaglandins Leukot Essent Fatty Acids. 1994 Dec;51(6):419–424. doi: 10.1016/0952-3278(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Martinolle J. P., Garcia-Villar R., More J., Bueno L. Evidence for mast cell, leukotriene and nitric oxide involvement in the regulation of the adrenoceptor number of inflamed small intestine in guinea pigs. Neurogastroenterol Motil. 1995 Sep;7(3):187–195. doi: 10.1111/j.1365-2982.1995.tb00224.x. [DOI] [PubMed] [Google Scholar]
- Martinolle J. P., Moré J., Dubech N., Garcia-Villar R. Inverse regulation of alpha- and beta-adrenoceptors during trinitrobenzenesulfonic acid (TNB)-induced inflammation in guinea-pig small intestine. Life Sci. 1993;52(18):1499–1508. doi: 10.1016/0024-3205(93)90112-g. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Sadowska-Krowicka H., Chotinaruemol S., Kakkis J. L., Clark D. A. Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther. 1993 Jan;264(1):11–16. [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Morteau O., Bueno L. Modèles animaux d'inflammation intestinale. Gastroenterol Clin Biol. 1995 Feb;19(2):204–214. [PubMed] [Google Scholar]
- Morteau O., More J., Pons L., Bueno L. Platelet-activating factor and interleukin 1 are involved in colonic dysmotility in experimental colitis in rats. Gastroenterology. 1993 Jan;104(1):47–56. doi: 10.1016/0016-5085(93)90834-y. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Lees C. J., Miller C. H., McCall C. E., Lewis J. C., Love S. H., Wykle R. L. Selective desensitization of neutrophils: further studies with 1-O-alkyl-sn-glycero-3-phosphocholine analogues. J Immunol. 1981 Aug;127(2):731–737. [PubMed] [Google Scholar]
- Rachmilewitz D., Eliakim R., Simon P., Ligumsky M., Karmeli F. Cytokines and platelet-activating factor in human inflamed colonic mucosa. Agents Actions. 1992;Spec No:C32–C36. [PubMed] [Google Scholar]
- Sethi A. K., Sarna S. K. Colonic motor activity in acute colitis in conscious dogs. Gastroenterology. 1991 Apr;100(4):954–963. doi: 10.1016/0016-5085(91)90269-q. [DOI] [PubMed] [Google Scholar]
- Snape W. J., Jr, Williams R., Hyman P. E. Defect in colonic smooth muscle contraction in patients with ulcerative colitis. Am J Physiol. 1991 Dec;261(6 Pt 1):G987–G991. doi: 10.1152/ajpgi.1991.261.6.G987. [DOI] [PubMed] [Google Scholar]
- Sobhani I., Hochlaf S., Denizot Y., Vissuzaine C., Rene E., Benveniste J., Lewin M. M., Mignon M. Raised concentrations of platelet activating factor in colonic mucosa of Crohn's disease patients. Gut. 1992 Sep;33(9):1220–1225. doi: 10.1136/gut.33.9.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivierge M., Alami N., Müller E., de Brum-Fernandes A. J., Rola-Pleszczynski M. Transcriptional modulation of platelet-activating factor receptor gene expression by cyclic AMP. J Biol Chem. 1993 Aug 15;268(23):17457–17462. [PubMed] [Google Scholar]
- Tokumura A., Yoshida J., Okasaka N., Fukuzawa K., Tsukatani H. Platelet aggregation induced by ether-linked phospholipids. 2. Mechanism of desensitization of rabbit platelets by platelet activating factor and reversibility of inhibitory actions of its antagonists. Thromb Res. 1987 Apr 1;46(1):153–161. doi: 10.1016/0049-3848(87)90215-5. [DOI] [PubMed] [Google Scholar]
- Wallace J. L. Release of platelet-activating factor (PAF) and accelerated healing induced by a PAF antagonist in an animal model of chronic colitis. Can J Physiol Pharmacol. 1988 Apr;66(4):422–425. doi: 10.1139/y88-071. [DOI] [PubMed] [Google Scholar]
- Zhou W., Javors M. A., Olson M. S. Platelet-activating factor as an intercellular signal in neutrophil-dependent platelet activation. J Immunol. 1992 Sep 1;149(5):1763–1769. [PubMed] [Google Scholar]