Abstract
Background—The plasma kallikrein-kinin (K-K) system is activated in acute and chronic relapsing intestinal inflammation induced in Lewis rats by intramural injection of exogenous bacterial components. Aims—To determine whether this effect is model specific, K-K system activation was investigated in a modified indomethacin induced enterocolitis model, as well as bradykinin 2 (B2) receptor distribution in the normal and acutely inflamed intestine. Methods—Lewis rats injected with daily sublethal doses of indomethacin for two days developed acute (two days) and chronic (14 days) intestinal inflammation. Plasma prekallikrein (amidolytic), high molecular weight kininogen (HK, coagulant) and cleavage of HK (western blot) were assayed to detect K-K activation. Results—Liver and spleen weights were significantly higher, and body weights and haematocrit values were significantly lower in the indomethacin group than in the control group. During both acute and chronic phases, rats displayed K-K system activation manifested by a significant decrease in plasma prekallikrein and HK functional levels, and by HK cleavage. Plasma T kininogen (a major acute phase protein) was significantly elevated. B2 receptors were identified in both normal and inflammatory intestine with more prominent specific immunohistochemical staining in the acutely inflamed tissue. Conclusions—K-K system activation occurs in association with both acute and chronic phases of intestinal injury, regardless of the triggering agent, suggesting that activation of this system is integrally involved in intestinal inflammation in genetically susceptible hosts. Localisation of B2 receptors across intestinal layers provides a structural basis for the kinin function in the intestine.
Keywords: plasma kallikrein; kininogen; bradykinin 2 receptor; enterocolitis; indomethacin; proteoglycan-polysaccharide
Full Text
The Full Text of this article is available as a PDF (291.2 KB).
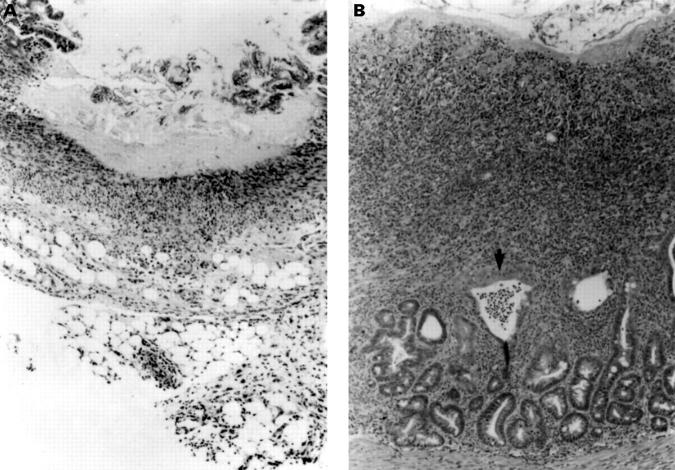
Figure 1 .
Histological evidence of enteritis induced by indomethacin in female Lewis rats. (A) At day 2, after subcutaneous injection of 7.5 mg/kg indomethacin for two days, a large ulcer with relatively bland necrosis of the entire mucosa is seen with severe serosal and mesenteric inflammation with neutrophils and macrophages (original magnification ×100). (B) At day 14, there is an extensive ulcer with an active mucosal exudate. A crypt abscess is visible (arrow) and glandular dysplasia with depletion of goblet cells. Transmural inflammation with thickening of the submucosa is evident. The infiltrate consists of both mononuclear cells and neutrophils with fibroblast proliferation and smooth muscle hypertrophy. Collagen indicative of early fibrosis is present (original magnification ×100).
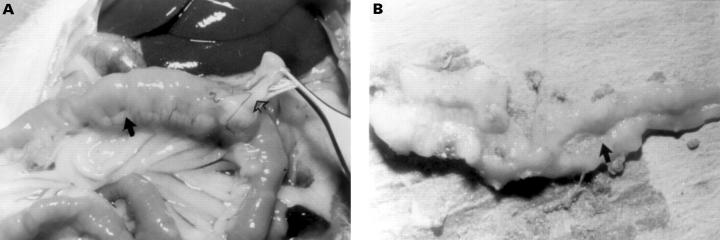
Figure 2 .
Gross appearance of intestinal inflammation in indomethacin treated Lewis rats. (A) Thickening of the bowel wall with creeping mesenteric fat (closed arrow) and adhesions (open arrow) is present at day 14 after injection of indomethacin on days 0 and 1. (B) Longitudinal ulcers (arrow) on the mesenteric border are seen in the mid small bowel at day 14.
Figure 3 .
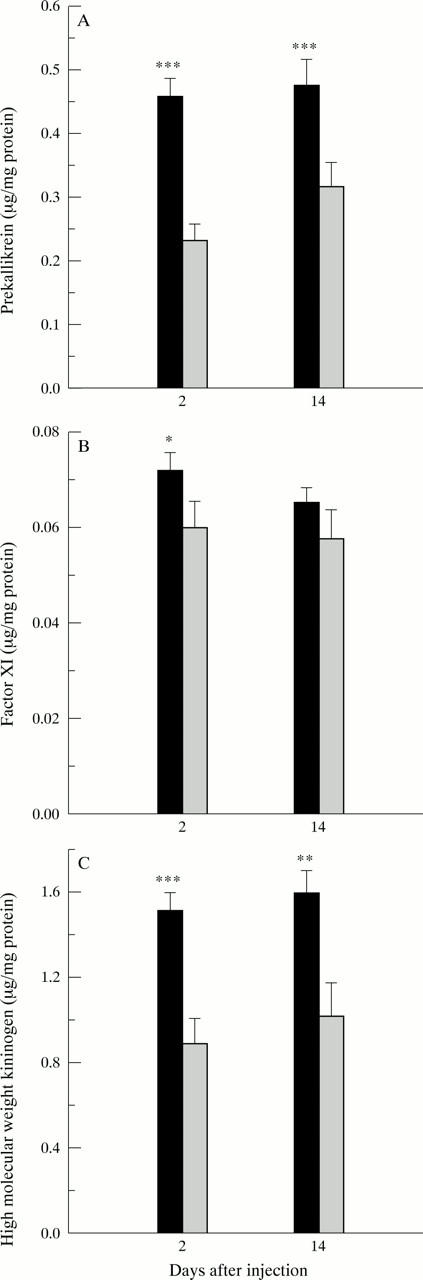
Functional assays of contact system proteins. (A) Prekallikrein; (B) factor XI; (C) high molecular weight kininogen. *p<0.05, **p<0.01, ***p<0.001. Solid bars indicate control groups; shaded bars indicate indomethacin treated groups.
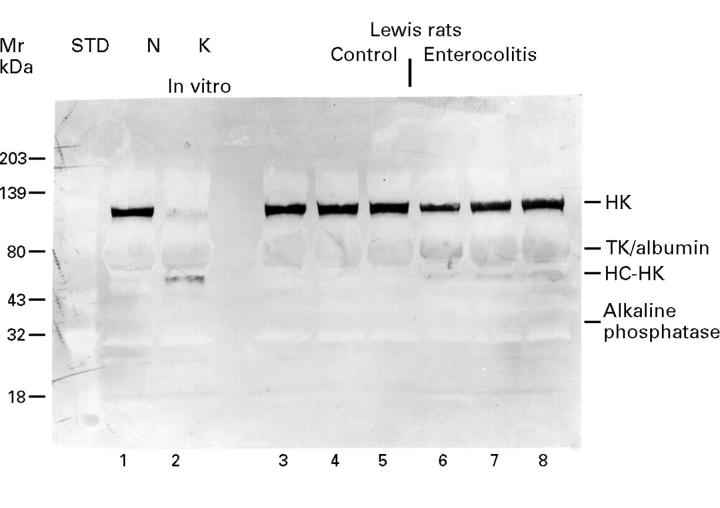
Figure 4 .
High molecular weight kininogen cleavage in Lewis rats treated with indomethacin shown by western blotting using anti-high molecular weight kininogen antibodies. Lane 1 shows pooled non-activated normal rat plasma (N), and lane 2 pooled rat plasma following exposure to kaolin (K). Lanes 3-5 represent the plasma samples of three control rats, while lanes 6-8 are plasma samples of rats 14 days after treatment with indomethacin. The 120 kDa band represents HK and is visualised across lanes 1 and 3-8, but is absent in lane 2. The broad band localised at about 68 kDa across all lanes indicates cross reacting T kininogen (TK) and albumin. The band situated between albumin and the 43 kDa standard, visualised on lane 2 and lanes 6-8, but not on lane 1 and lanes 3-5, represents the heavy chain of high molecular weight kininogen (HC-HK, 62 kDa). The delicate band situated just above 32 kDa, standard across all lanes, represents a non-specific reaction with alkaline phosphatase that hydrolyses the BCIP/NBT substrate used to develop the colour.
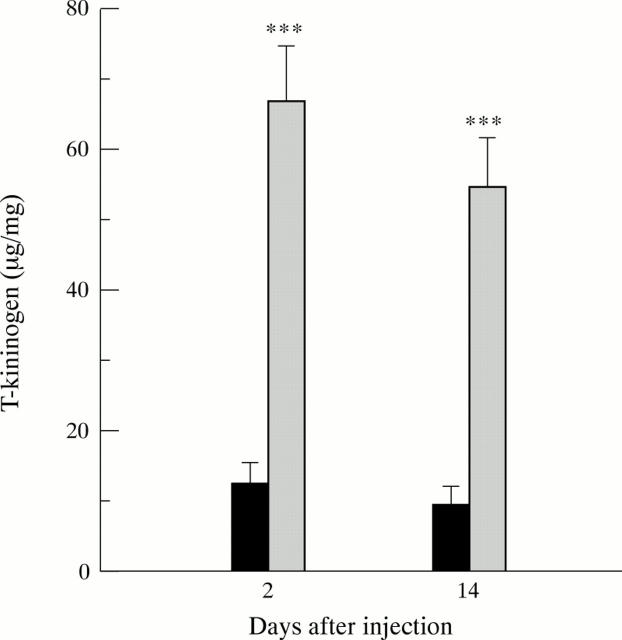
Figure 5 .
Plasma T kininogen levels during acute phase (two days) and chronic phase (14 days) of inflammation after indomethacin injection (mean (SEM)). T kininogen levels are expressed as µg/mg plasma protein. ***p<0.001. Solid bars, buffer control rats; shaded bars, rats treated with indomethacin.
Figure 6 .
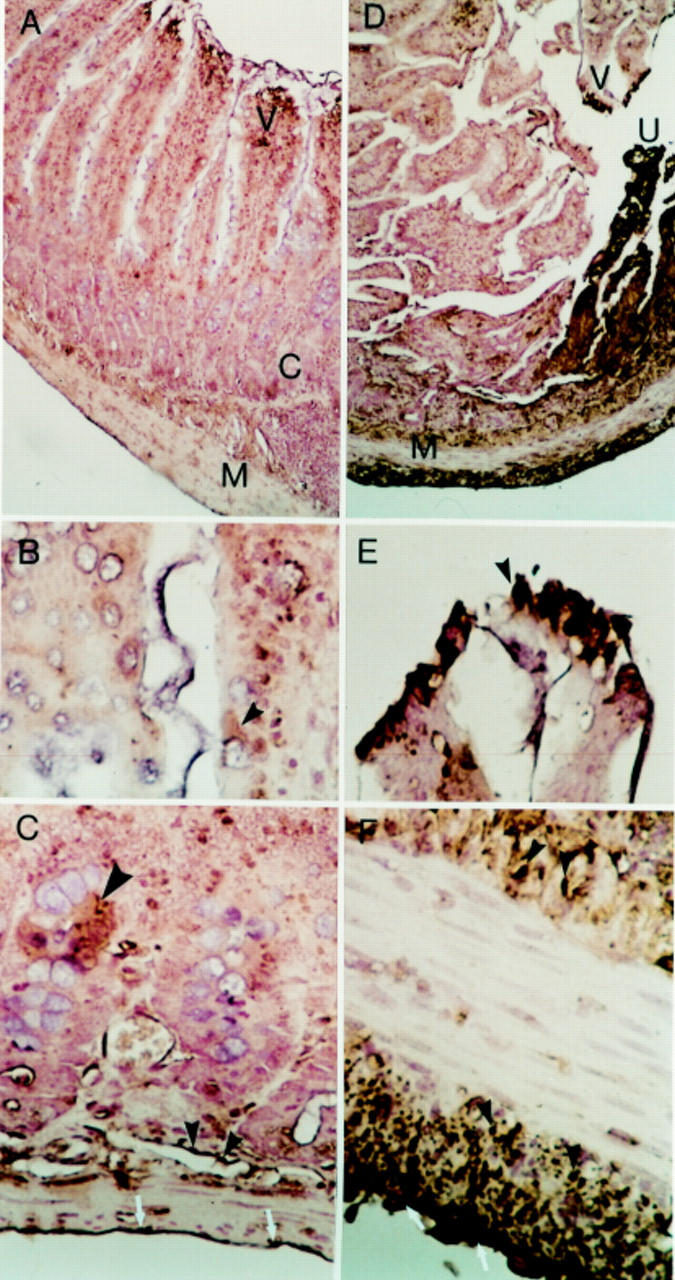
Immunolocalisation of the kinin B2 receptor in rat intestine on day 2. A-C: Rat normal control jejunum (buffer treated). (A) Some focal staining positive for B2 receptor in the villi—V, occasionally in the muscularis mucosa below the crypts—C, in the muscular layer—M, and in the serosa. Original magnification ×100. (B) Intestinal villi. Focal weak immunostaining in the surface of occasional epithelial cells and in cytoplasm (arrow). Original magnification ×400. (C) Some focal immunostaining, cluster of epithelial cells in crypts (large arrow), in the muscular layers (small solid arrows), and in serosal surface (white arrows). Original magnification ×400. D-F: Rat inflamed intestine (day 2 after injection of indomethacin). (D) Focal immunostaining in the villi—V, especially near the area of ulceration - U, in the internal and external muscular layer—M, and in the serosa. Original magnification ×100. (E) Villus near area of ulceration showing strong staining in the epithelial, probably absorptive cells (arrow). Original magnification ×400. (F) Prominent immunostaining in the smooth muscle cells of the muscularis mucosa (solid arrows) and in the external muscular layer (solid arrows). On the serosal surface, staining is also present (white arrows). Original magnification ×400.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Damas J., Calay G., Renard C., Remacle-Volon G., Bourdon V. Quantification of rat T-kininogen using immunological methods. Application to inflammatory processes. Biochem Pharmacol. 1989 May 15;38(10):1569–1575. doi: 10.1016/0006-2952(89)90303-1. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. Enteropathy induced by non-steroidal anti-inflammatory drugs. BMJ. 1989 Jun 10;298(6687):1539–1540. doi: 10.1136/bmj.298.6687.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K., Peters T. J. Experimental non-steroidal anti-inflammatory drug-induced enteropathy in the rat: similarities to inflammatory bowel disease and effect of thromboxane synthetase inhibitors. Gut. 1990 Dec;31(12):1358–1364. doi: 10.1136/gut.31.12.1358. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bhoola K. D., Figueroa C. D., Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992 Mar;44(1):1–80. [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Smith T., Prouse P., Williams P., Smethurst P., Delacey G., Gumpel M. J., Levi A. J. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology. 1987 Sep;93(3):480–489. doi: 10.1016/0016-5085(87)90909-7. [DOI] [PubMed] [Google Scholar]
- Blais C., Jr, Couture R., Drapeau G., Colman R. W., Adam A. Involvement of endogenous kinins in the pathogenesis of peptidoglycan-induced arthritis in the Lewis rat. Arthritis Rheum. 1997 Jul;40(7):1327–1333. doi: 10.1002/1529-0131(199707)40:7<1327::AID-ART18>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Colman R. W., Bagdasarian A., Talamo R. C., Scott C. F., Seavey M., Guimaraes J. A., Pierce J. V., Kaplan A. P. Williams trait. Human kininogen deficiency with diminished levels of plasminogen proactivator and prekallikrein associated with abnormalities of the Hageman factor-dependent pathways. J Clin Invest. 1975 Dec;56(6):1650–1662. doi: 10.1172/JCI108247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman R. W., Flores D. N., De La Cadena R. A., Scott C. F., Cousens L., Barr P. J., Hoffman I. B., Kueppers F., Fisher D., Idell S. Recombinant alpha 1-antitrypsin Pittsburgh attenuates experimental gram-negative septicemia. Am J Pathol. 1988 Feb;130(2):418–426. [PMC free article] [PubMed] [Google Scholar]
- De La Cadena R. A., Scott C. F., Colman R. W. Evaluation of a microassay for human plasma prekallikrein. J Lab Clin Med. 1987 May;109(5):601–607. [PubMed] [Google Scholar]
- Elson C. O., Sartor R. B., Tennyson G. S., Riddell R. H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995 Oct;109(4):1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Fang W. F., Broughton A., Jacobson E. D. Indomethacin-induced intestinal inflammation. Am J Dig Dis. 1977 Sep;22(9):749–760. doi: 10.1007/BF01694504. [DOI] [PubMed] [Google Scholar]
- Figueroa C. D., Gonzalez C. B., Grigoriev S., Abd Alla S. A., Haasemann M., Jarnagin K., Müller-Esterl W. Probing for the bradykinin B2 receptor in rat kidney by anti-peptide and anti-ligand antibodies. J Histochem Cytochem. 1995 Feb;43(2):137–148. doi: 10.1177/43.2.7822771. [DOI] [PubMed] [Google Scholar]
- Gaginella T. S., Kachur J. F. Kinins as mediators of intestinal secretion. Am J Physiol. 1989 Jan;256(1 Pt 1):G1–15. doi: 10.1152/ajpgi.1989.256.1.G1. [DOI] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Hock J., Vogel R., Linke R. P., Müller-Esterl W. High molecular weight kininogen-binding site of prekallikrein probed by monoclonal antibodies. J Biol Chem. 1990 Jul 15;265(20):12005–12011. [PubMed] [Google Scholar]
- Hollander D. The intestinal permeability barrier. A hypothesis as to its regulation and involvement in Crohn's disease. Scand J Gastroenterol. 1992 Sep;27(9):721–726. doi: 10.3109/00365529209011172. [DOI] [PubMed] [Google Scholar]
- Kaufmann H. J., Taubin H. L. Nonsteroidal anti-inflammatory drugs activate quiescent inflammatory bowel disease. Ann Intern Med. 1987 Oct;107(4):513–516. doi: 10.7326/0003-4819-107-4-513. [DOI] [PubMed] [Google Scholar]
- Kent T. H., Cardelli R. M., Stamler F. W. Small intestinal ulcers and intestinal flora in rats given indomethacin. Am J Pathol. 1969 Feb;54(2):237–249. [PMC free article] [PubMed] [Google Scholar]
- Means R. T., Jr, Krantz S. B. Progress in understanding the pathogenesis of the anemia of chronic disease. Blood. 1992 Oct 1;80(7):1639–1647. [PubMed] [Google Scholar]
- Miura S., Suematsu M., Tanaka S., Nagata H., Houzawa S., Suzuki M., Kurose I., Serizawa H., Tsuchiya M. Microcirculatory disturbance in indomethacin-induced intestinal ulcer. Am J Physiol. 1991 Aug;261(2 Pt 1):G213–G219. doi: 10.1152/ajpgi.1991.261.2.G213. [DOI] [PubMed] [Google Scholar]
- PROCTOR R. R., RAPAPORT S. I. The partial thromboplastin time with kaolin. A simple screening test for first stage plasma clotting factor deficiencies. Am J Clin Pathol. 1961 Sep;36:212–219. doi: 10.1093/ajcp/36.3.212. [DOI] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Pixley R. A., De La Cadena R., Page J. D., Kaufman N., Wyshock E. G., Chang A., Taylor F. B., Jr, Colman R. W. The contact system contributes to hypotension but not disseminated intravascular coagulation in lethal bacteremia. In vivo use of a monoclonal anti-factor XII antibody to block contact activation in baboons. J Clin Invest. 1993 Jan;91(1):61–68. doi: 10.1172/JCI116201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pixley R. A., Zellis S., Bankes P., DeLa Cadena R. A., Page J. D., Scott C. F., Kappelmayer J., Wyshock E. G., Kelly J. J., Colman R. W. Prognostic value of assessing contact system activation and factor V in systemic inflammatory response syndrome. Crit Care Med. 1995 Jan;23(1):41–51. doi: 10.1097/00003246-199501000-00010. [DOI] [PubMed] [Google Scholar]
- Rath H. C., Herfarth H. H., Ikeda J. S., Grenther W. B., Hamm T. E., Jr, Balish E., Taurog J. D., Hammer R. E., Wilson K. H., Sartor R. B. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996 Aug 15;98(4):945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern J. S., Blair A. J., 3rd, Lee E., Feldman M. Gastrointestinal ulcer formation in rabbits immunized with prostaglandin E2. Gastroenterology. 1987 Oct;93(4):744–752. doi: 10.1016/0016-5085(87)90436-7. [DOI] [PubMed] [Google Scholar]
- Robert A. An intestinal disease produced experimentally by a prostaglandin deficiency. Gastroenterology. 1975 Oct;69(4):1045–1047. [PubMed] [Google Scholar]
- Sartor R. B. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn's disease. Gastroenterol Clin North Am. 1995 Sep;24(3):475–507. [PubMed] [Google Scholar]
- Sartor R. B., DeLa Cadena R. A., Green K. D., Stadnicki A., Davis S. W., Schwab J. H., Adam A. A., Raymond P., Colman R. W. Selective kallikrein-kinin system activation in inbred rats differentially susceptible to granulomatous enterocolitis. Gastroenterology. 1996 May;110(5):1467–1481. doi: 10.1053/gast.1996.v110.pm8613052. [DOI] [PubMed] [Google Scholar]
- Schapira M., Despland E., Scott C. F., Boxer L. A., Colman R. W. Purified human plasma kallikrein aggregates human blood neutrophils. J Clin Invest. 1982 May;69(5):1199–1202. doi: 10.1172/JCI110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G., Tsykin A., Aldred A. R., Thomas T., Fung W. P., Dickson P. W., Cole T., Birch H., De Jong F. A., Milland J. The acute phase response in the rodent. Ann N Y Acad Sci. 1989;557:61–86. doi: 10.1111/j.1749-6632.1989.tb24000.x. [DOI] [PubMed] [Google Scholar]
- Schreiber G., Tsykin A., Aldred A. R., Thomas T., Fung W. P., Dickson P. W., Cole T., Birch H., De Jong F. A., Milland J. The acute phase response in the rodent. Ann N Y Acad Sci. 1989;557:61–86. doi: 10.1111/j.1749-6632.1989.tb24000.x. [DOI] [PubMed] [Google Scholar]
- Schwab J. H. Phlogistic properties of peptidoglycan-polysaccharide polymers from cell walls of pathogenic and normal-flora bacteria which colonize humans. Infect Immun. 1993 Nov;61(11):4535–4539. doi: 10.1128/iai.61.11.4535-4539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. F., Colman R. W. A simple and accurate microplate assay for the determination of factor XI in plasma. J Lab Clin Med. 1988 Jun;111(6):708–714. [PubMed] [Google Scholar]
- Stadnicki A., DeLa Cadena R. A., Sartor R. B., Bender D., Kettner C. A., Rath H. C., Adam A., Colman R. W. Selective plasma kallikrein inhibitor attenuates acute intestinal inflammation in Lewis rat. Dig Dis Sci. 1996 May;41(5):912–920. doi: 10.1007/BF02091530. [DOI] [PubMed] [Google Scholar]
- Stadnicki A., Gonciarz M., Niewiarowski T. J., Hartleb J., Rudnicki M., Merrell N. B., Dela Cadena R. A., Colman R. W. Activation of plasma contact and coagulation systems and neutrophils in the active phase of ulcerative colitis. Dig Dis Sci. 1997 Nov;42(11):2356–2366. doi: 10.1023/a:1018891323205. [DOI] [PubMed] [Google Scholar]
- Stadnicki A., Sartor R. B., Janardham R., Majluf-Cruz A., Kettner C. A., Adam A. A., Colman R. W. Specific inhibition of plasma kallikrein modulates chronic granulomatous intestinal and systemic inflammation in genetically susceptible rats. FASEB J. 1998 Mar;12(3):325–333. doi: 10.1096/fasebj.12.3.325. [DOI] [PubMed] [Google Scholar]
- Wachtfogel Y. T., Kucich U., James H. L., Scott C. F., Schapira M., Zimmerman M., Cohen A. B., Colman R. W. Human plasma kallikrein releases neutrophil elastase during blood coagulation. J Clin Invest. 1983 Nov;72(5):1672–1677. doi: 10.1172/JCI111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle B. J. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology. 1981 Jan;80(1):94–98. [PubMed] [Google Scholar]
- Yamada T., Deitch E., Specian R. D., Perry M. A., Sartor R. B., Grisham M. B. Mechanisms of acute and chronic intestinal inflammation induced by indomethacin. Inflammation. 1993 Dec;17(6):641–662. doi: 10.1007/BF00920471. [DOI] [PubMed] [Google Scholar]
- Zimmerli W., Huber I., Bouma B. N., Lämmle B. Purified human plasma kallikrein does not stimulate but primes neutrophils for superoxide production. Thromb Haemost. 1989 Dec 29;62(4):1121–1125. [PubMed] [Google Scholar]