Abstract
Background—Clarithromycin is one of the most important antibiotics for Helicobacter pylori eradication. However, 5-10% of strains are reported to be resistant. It has been shown that one point mutation in the 23S rRNA gene is associated with resistance to clarithromycin. Aims—To establish a polymerase chain reaction (PCR) system which amplifies a segment of the 23S rRNA gene containing the mutation points with primers specific for H pylori, so that H pylori infection and the mutation associated with clarithromycin resistance can be examined simultaneously. Methods—To detect H pylori infection and the mutation simultaneously, primers specific for the H pylori 23S rRNA gene were designed based on sequence conservation among H pylori strains and sequence specificity as compared with other bacteria. DNA from 57 cultured strains and from 39 gastric juice samples was amplified in the seminested 23S rRNA PCR. Clinical applicability was evaluated in 85patients. Results—DNA samples from 57 cultured strains were all amplified. The novel assay and the urease A PCR agreed in 37/39 gastric juice samples with no false positives. The assay did not amplify the DNA of bacteria other than H pylori. Eight of 85 samples had the mutation before treatment. In clarithromycin based treatment, eradication was achieved in 2/5 (40%) with the mutation and 29/34 (85%) without the mutation. Conclusion—The assay using gastric juice is quick (within 12 hours) and non-invasive (endoscopy not required), enabling rapid initiation of appropriate antibiotic treatment.
Keywords: Helicobacter pylori; eradication; clarithromycin; resistance; point mutation
Full Text
The Full Text of this article is available as a PDF (128.4 KB).
Figure 1 .

23S rRNA gene sequence in seven H pylori strains. 23S reported was the sequence reported in GenBank (accession number U27270). Colons denote identity.
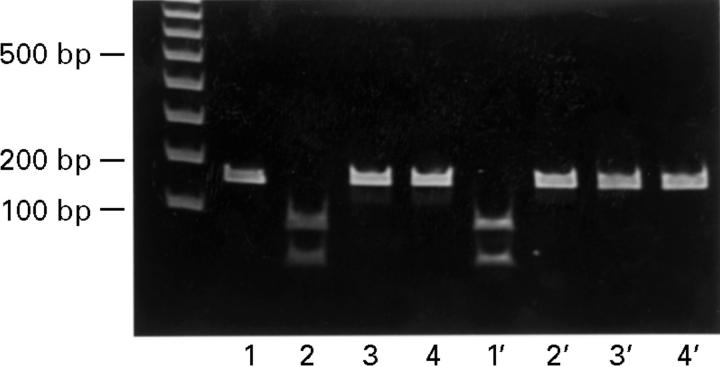
Figure 2 .
Detection of A to G mutation. PCR products from four strains were digested with MboII (lanes 1-4) and with BsaI (lanes 1'-4'). Strain 2 had the A to G mutation at 2143 (lane 2), strain 1 had the A to G mutation at 2144 (lane 1'), and strains 3 and 4 had no mutations at either 2143 or 2144.
Figure 3 .
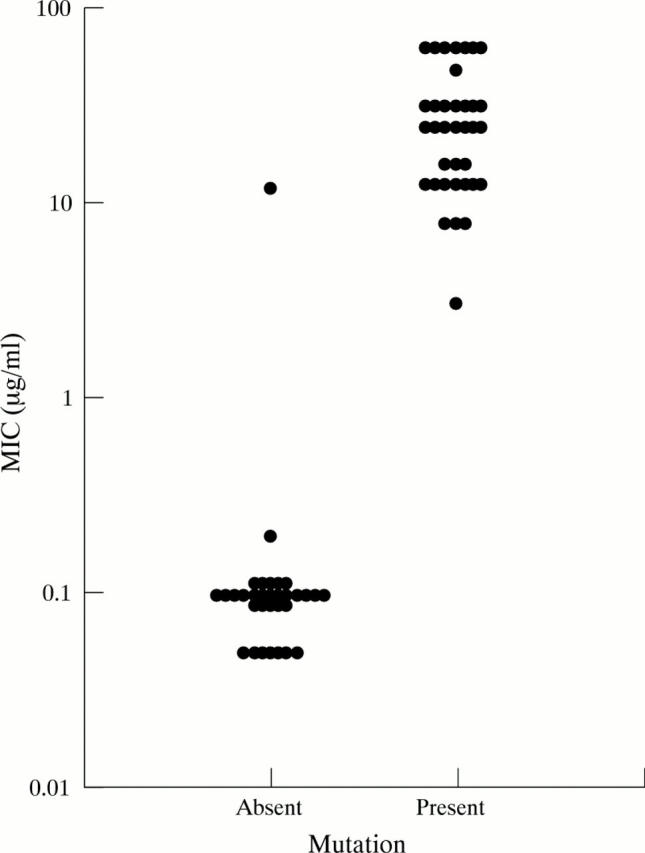
Relation between the A to G mutation at 2143/2144 and MIC. All strains with the mutation at 2143/2144 were resistant to clarithromycin by MIC. One strain was resistant by MIC but had no mutation. Those without the mutations were susceptible to clarithromycin by MIC.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiba N. Omeprazole and clarithromycin with and without metronidazole for the eradication of Helicobacter pylori. Am J Gastroenterol. 1996 Oct;91(10):2139–2143. [PubMed] [Google Scholar]
- Debets-Ossenkopp Y. J., Sparrius M., Kusters J. G., Kolkman J. J., Vandenbroucke-Grauls C. M. Mechanism of clarithromycin resistance in clinical isolates of Helicobacter pylori. FEMS Microbiol Lett. 1996 Aug 15;142(1):37–42. doi: 10.1111/j.1574-6968.1996.tb08404.x. [DOI] [PubMed] [Google Scholar]
- Gotoh A., Kawakami Y., Akahane T., Akamatsu T., Shimizu T., Kiyosawa K., Katsuyama T. Susceptibility of Helicobacter pylori isolates against agents commonly administered for eradication therapy and the efficacy of chemotherapy. Microbiol Immunol. 1997;41(1):7–12. doi: 10.1111/j.1348-0421.1997.tb01166.x. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Lew G. M., Klein P. D., Evans D. G., Evans D. J., Jr, Saeed Z. A., Malaty H. M. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992 May 1;116(9):705–708. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- Kawamata O., Yoshida H., Hirota K., Yoshida A., Kawaguchi R., Shiratori Y., Omata M. Nested-polymerase chain reaction for the detection of Helicobacter pylori infection with novel primers designed by sequence analysis of urease A gene in clinically isolated bacterial strains. Biochem Biophys Res Commun. 1996 Feb 6;219(1):266–272. doi: 10.1006/bbrc.1996.0216. [DOI] [PubMed] [Google Scholar]
- Labenz J., Stolte M., Rühl G. H., Becker T., Tillenburg B., Sollböhmer M., Börsch G. One-week low-dose triple therapy for the eradication of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1995 Jan;7(1):9–11. [PubMed] [Google Scholar]
- Lind T., Veldhuyzen van Zanten S., Unge P., Spiller R., Bayerdörffer E., O'Morain C., Bardhan K. D., Bradette M., Chiba N., Wrangstadh M. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter. 1996 Sep;1(3):138–144. doi: 10.1111/j.1523-5378.1996.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Midolo P. D., Lambert J. R., Turnidge J. Metronidazole resistance: a predictor of failure of Helicobacter pylori eradication by triple therapy. J Gastroenterol Hepatol. 1996 Mar;11(3):290–292. doi: 10.1111/j.1440-1746.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Penston J. G., McColl K. E. Eradication of Helicobacter pylori: an objective assessment of current therapies. Br J Clin Pharmacol. 1997 Mar;43(3):223–243. doi: 10.1046/j.1365-2125.1997.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone G. G., Shortridge D., Versalovic J., Beyer J., Flamm R. K., Graham D. Y., Ghoneim A. T., Tanaka S. K. A PCR-oligonucleotide ligation assay to determine the prevalence of 23S rRNA gene mutations in clarithromycin-resistant Helicobacter pylori. Antimicrob Agents Chemother. 1997 Mar;41(3):712–714. doi: 10.1128/aac.41.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Versalovic J., Shortridge D., Kibler K., Griffy M. V., Beyer J., Flamm R. K., Tanaka S. K., Graham D. Y., Go M. F. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1996 Feb;40(2):477–480. doi: 10.1128/aac.40.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. H., Peterson W. L. The treatment of Helicobacter pylori infection in the management of peptic ulcer disease. N Engl J Med. 1995 Oct 12;333(15):984–991. doi: 10.1056/NEJM199510123331508. [DOI] [PubMed] [Google Scholar]
- Weldon M. J., Broadbent A., Chambers S., Mistry R., Ranganath L., Gould S. R. A seven-day Helicobacter pylori treatment regimen using clarithromycin, omeprazole and tripotassium dicitrato bismuthate. Aliment Pharmacol Ther. 1996 Jun;10(3):279–283. doi: 10.1111/j.0953-0673.1996.00279.x. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Hirota K., Shiratori Y., Nihei T., Amano S., Yoshida A., Kawamata O., Omata M. Use of a gastric juice-based PCR assay to detect Helicobacter pylori infection in culture-negative patients. J Clin Microbiol. 1998 Jan;36(1):317–320. doi: 10.1128/jcm.36.1.317-320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zwet A. A., de Boer W. A., Schneeberger P. M., Weel J., Jansz A. R., Thijs J. C. Prevalence of primary Helicobacter pylori resistance to metronidazole and clarithromycin in The Netherlands. Eur J Clin Microbiol Infect Dis. 1996 Nov;15(11):861–864. doi: 10.1007/BF01691216. [DOI] [PubMed] [Google Scholar]