Abstract
Background—Ectopic protease activation, microcirculatory changes, and leucocyte activation are the main events in the pathogenesis of acute pancreatitis. Nitric oxide (NO) is known to be a key mediator in the normal and inflamed pancreas. Aims—To investigate the targets on which NO exerts its effect in caerulein induced pancreatitis. Methods—Acute pancreatitis was induced in rats which additionally received either the NO synthase substrate, L-arginine; the NO donor, sodium nitroprusside; or the NO synthase inhibitor, N-nitro-L-arginine methyl ester (L-NAME). At six hours, pancreatic injury (oedema, leucocyte content, ectopic trypsinogen activation) was analysed and pancreatic oxygenation and perfusion were determined. A direct influence of NO on amylase secretion and trypsinogen activation was evaluated separately in vitro. Results—Both NO donors reduced the grade of inflammation. L-NAME increased the severity of inflammation, while decreasing pancreatic tissue oxygenation. Although neither amylase secretion nor intracellular trypsinogen activation in caerulein stimulated pancreatic acini was influenced by either NO donors or inhibitors, both NO donors decreased intrapancreatic trypsinogen activation peptide (TAP) and pancreatic oedema in vivo, and L-NAME increased TAP. Conclusions—NO protects against injury caused by pancreatitis in the intact animal but has no discernible effect on isolated acini. It is likely that in pancreatitis NO acts indirectly via microcirculatory changes, including inhibition of leucocyte activation and preservation of capillary perfusion.
Keywords: acute pancreatitis; nitric oxide; microcirculation; leucocytes; pancreatic secretion
Full Text
The Full Text of this article is available as a PDF (134.0 KB).
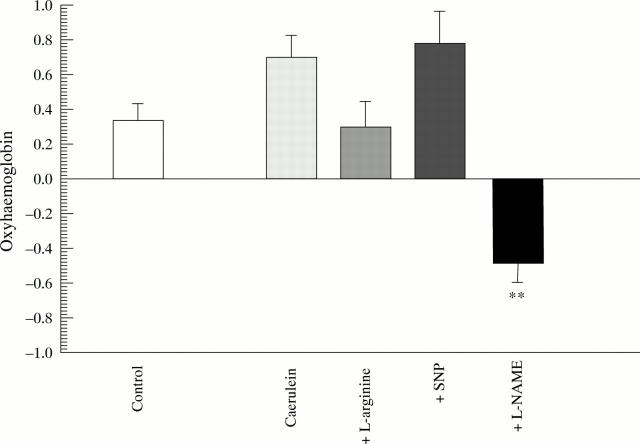
Figure 1 .
Diffuse reflectance spectroscopy. Difference in oxyhaemoglobin content in pancreatic tissue between baseline (0 hours) and end point (6 hours) measurements. **p<0.01 compared with caerulein.
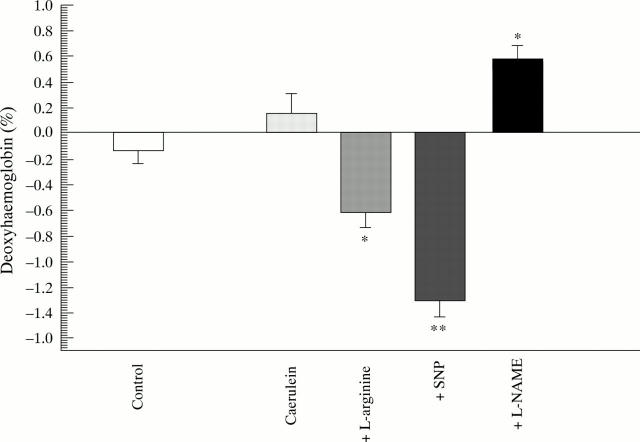
Figure 2 .
Diffuse reflectance spectroscopy. Difference in deoxyhaemoglobin content in pancreatic tissue between baseline (0 hours) and end point (6 hours) measurements. *p<0.05, **p<0.01 compared with caerulein.
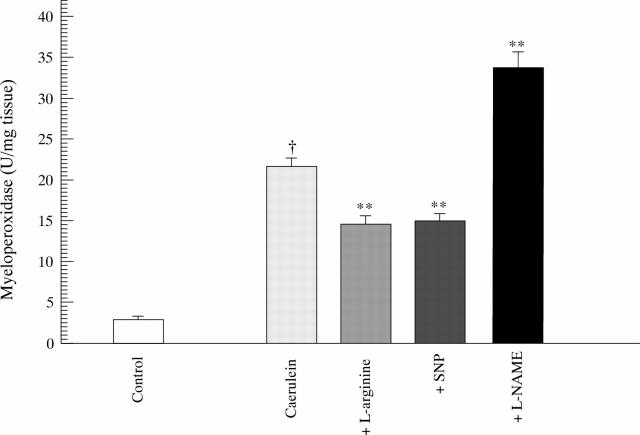
Figure 3 .
Leucocyte infiltration of pancreatic tissue as evaluated by myeloperoxidase activity. **p<0.01 compared with caerulein; †p<0.05 compared with control.
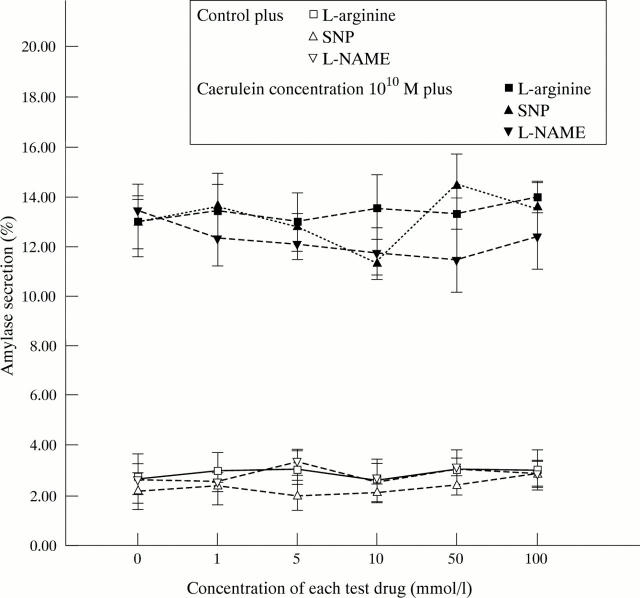
Figure 4 .
Influence of NO donors and inhibitors on amylase secretion of unstimulated versus secretory stimulated pancreatic acini.
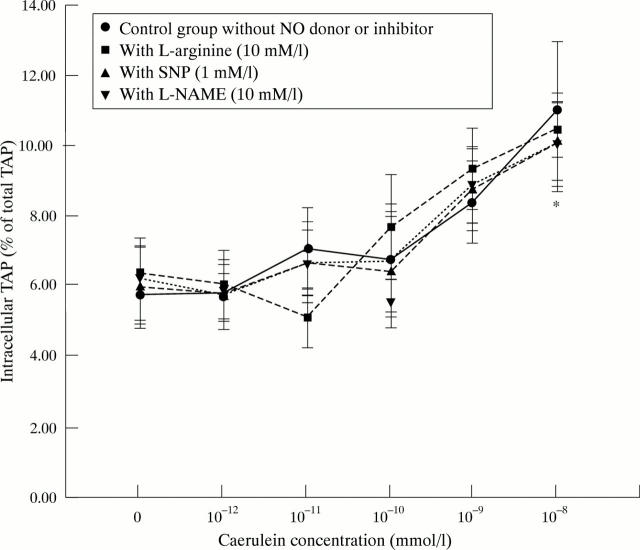
Figure 5 .
Influence of NO donors and inhibitors on intracellular TAP formation in pancreatic acinar cells (as a measurement of intracellular trypsinogen activation) in response to different grades of secretory stimuli with caerulein. *p<0.05 compared with control group and with caerulein 10−12 M.
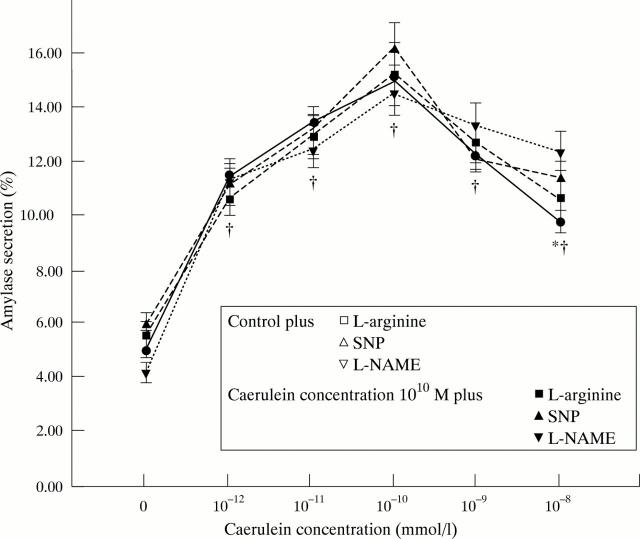
Figure 6 .
Influence of NO donors and inhibitors on amylase secretion by pancreatic acinar cells in response to different grades of secretory stimuli with caerulein.*p<0.05 compared with caerulein 10−12 M; †p<0.05 compared with control.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Shimosegawa T., Satoh A., Abe R., Kikuchi Y., Koizumi M., Toyota T. Nitric oxide modulates pancreatic edema formation in rat caerulein-induced pancreatitis. J Gastroenterol. 1995 Oct;30(5):636–642. doi: 10.1007/BF02367791. [DOI] [PubMed] [Google Scholar]
- Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982 Mar;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Bruzzone R., Halban P. A., Gjinovci A., Trimble E. R. A new, rapid, method for preparation of dispersed pancreatic acini. Biochem J. 1985 Mar 1;226(2):621–624. doi: 10.1042/bj2260621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceska M., Birath K., Brown B. A new and rapid method for the clinical determination of alpha-amylase activities in human serum and urine. Optimal conditions. Clin Chim Acta. 1969 Dec;26(3):437–444. doi: 10.1016/0009-8981(69)90071-0. [DOI] [PubMed] [Google Scholar]
- Clancy R. M., Leszczynska-Piziak J., Abramson S. B. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992 Sep;90(3):1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowski A., Gabryelewicz A. Nitric oxide contributes to multiorgan oxidative stress in acute experimental pancreatitis. Scand J Gastroenterol. 1994 Oct;29(10):943–948. doi: 10.3109/00365529409094868. [DOI] [PubMed] [Google Scholar]
- Fernández-del Castillo C., Schmidt J., Warshaw A. L., Rattner D. W. Interstitial protease activation is the central event in progression to necrotizing pancreatitis. Surgery. 1994 Sep;116(3):497–504. [PubMed] [Google Scholar]
- Gaboury J., Woodman R. C., Granger D. N., Reinhardt P., Kubes P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am J Physiol. 1993 Sep;265(3 Pt 2):H862–H867. doi: 10.1152/ajpheart.1993.265.3.H862. [DOI] [PubMed] [Google Scholar]
- Gudgeon A. M., Heath D. I., Hurley P., Jehanli A., Patel G., Wilson C., Shenkin A., Austen B. M., Imrie C. W., Hermon-Taylor J. Trypsinogen activation peptides assay in the early prediction of severity of acute pancreatitis. Lancet. 1990 Jan 6;335(8680):4–8. doi: 10.1016/0140-6736(90)90135-r. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hutcheson I. R., Whittle B. J., Boughton-Smith N. K. Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol. 1990 Dec;101(4):815–820. doi: 10.1111/j.1476-5381.1990.tb14163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar E., Messmer K., Warshaw A. L., Herfarth C. Pancreatic ischaemia in experimental acute pancreatitis: mechanism, significance and therapy. Br J Surg. 1990 Nov;77(11):1205–1210. doi: 10.1002/bjs.1800771104. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Waltersdorph A. M., Rosen H. Antimicrobial activity of myeloperoxidase. Methods Enzymol. 1984;105:399–403. doi: 10.1016/s0076-6879(84)05055-2. [DOI] [PubMed] [Google Scholar]
- Knoefel W. T., Kollias N., Rattner D. W., Nishioka N. S., Warshaw A. L. Reflectance spectroscopy of pancreatic microcirculation. J Appl Physiol (1985) 1996 Jan;80(1):116–123. doi: 10.1152/jappl.1996.80.1.116. [DOI] [PubMed] [Google Scholar]
- Knoefel W. T., Kollias N., Warshaw A. L., Waldner H., Nishioka N. S., Rattner D. W. Pancreatic microcirculatory changes in experimental pancreatitis of graded severity in the rat. Surgery. 1994 Nov;116(5):904–913. [PubMed] [Google Scholar]
- Konturek S. J., Bilski J., Konturek P. K., Cieszkowski M., Pawlik W. Role of endogenous nitric oxide in the control of canine pancreatic secretion and blood flow. Gastroenterology. 1993 Mar;104(3):896–902. doi: 10.1016/0016-5085(93)91028-g. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Szlachcic A., Dembinski A., Warzecha Z., Jaworek J., Stachura J. Nitric oxide in pancreatic secretion and hormone-induced pancreatitis in rats. Int J Pancreatol. 1994 Feb;15(1):19–28. doi: 10.1007/BF02924384. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampel M., Kern H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Leach S. D., Modlin I. M., Scheele G. A., Gorelick F. S. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J Clin Invest. 1991 Jan;87(1):362–366. doi: 10.1172/JCI114995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Nakano I., Yamaguchi H., Ito T., Goto M., Koyanagi S., Kinjoh M., Nawata H. Protective effect of nitric oxide on development of acute pancreatitis in rats. Dig Dis Sci. 1995 Oct;40(10):2162–2169. doi: 10.1007/BF02209000. [DOI] [PubMed] [Google Scholar]
- Menozzi D., Sato S., Jensen R. T., Gardner J. D. Cyclic GMP does not inhibit protein kinase C-mediated enzyme secretion in rat pancreatic acini. J Biol Chem. 1989 Jan 15;264(2):995–999. [PubMed] [Google Scholar]
- Molero X., Guarner F., Salas A., Mourelle M., Puig V., Malagelada J. R. Nitric oxide modulates pancreatic basal secretion and response to cerulein in the rat: effects in acute pancreatitis. Gastroenterology. 1995 Jun;108(6):1855–1862. doi: 10.1016/0016-5085(95)90150-7. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Morikawa M., Inoue M., Tokumaru S., Kogo H. Enhancing and inhibitory effects of nitric oxide on superoxide anion generation in human polymorphonuclear leukocytes. Br J Pharmacol. 1995 Aug;115(7):1302–1306. doi: 10.1111/j.1476-5381.1995.tb15040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanji A. A., Greenberg S. S., Tahan S. R., Fogt F., Loscalzo J., Sadrzadeh S. M., Xie J., Stamler J. S. Nitric oxide production in experimental alcoholic liver disease in the rat: role in protection from injury. Gastroenterology. 1995 Sep;109(3):899–907. doi: 10.1016/0016-5085(95)90400-x. [DOI] [PubMed] [Google Scholar]
- Norman J. G., Fink G., Franz M., Guffey J., Carter G., Davison B., Sexton C., Glaccum M. Active interleukin-1 receptor required for maximal progression of acute pancreatitis. Ann Surg. 1996 Feb;223(2):163–169. doi: 10.1097/00000658-199602000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H. Fatal pancreatitis, a consequence of excessive leukocyte stimulation? Int J Pancreatol. 1988 Mar;3(2-3):105–112. doi: 10.1007/BF02798921. [DOI] [PubMed] [Google Scholar]
- Satoh A., Shimosegawa T., Abe T., Kikuchi Y., Abe R., Koizumi M., Toyota T. Role of nitric oxide in the pancreatic blood flow response to caerulein. Pancreas. 1994 Sep;9(5):574–579. doi: 10.1097/00006676-199409000-00006. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Fernández-del Castillo C., Rattner D. W., Lewandrowski K., Compton C. C., Warshaw A. L. Trypsinogen-activation peptides in experimental rat pancreatitis: prognostic implications and histopathologic correlates. Gastroenterology. 1992 Sep;103(3):1009–1016. doi: 10.1016/0016-5085(92)90036-x. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Lewandrowsi K., Warshaw A. L., Compton C. C., Rattner D. W. Morphometric characteristics and homogeneity of a new model of acute pancreatitis in the rat. Int J Pancreatol. 1992 Aug;12(1):41–51. doi: 10.1007/BF02927069. [DOI] [PubMed] [Google Scholar]
- Schoenberg M. H., Büchler M., Gaspar M., Stinner A., Younes M., Melzner I., Bültmann B., Beger H. G. Oxygen free radicals in acute pancreatitis of the rat. Gut. 1990 Oct;31(10):1138–1143. doi: 10.1136/gut.31.10.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimosegawa T., Abe T., Satoh A., Abe R., Kikuchi Y., Koizumi M., Toyota T. NADPH-diaphorase activity in neurons of the mammalian pancreas: coexpression with vasoactive intestinal polypeptide. Gastroenterology. 1993 Oct;105(4):999–1008. doi: 10.1016/0016-5085(93)90942-6. [DOI] [PubMed] [Google Scholar]
- Tsukahara Y., Horita Y., Anan K., Morisaki T., Tanaka M., Torisu M. Role of nitric oxide derived from alveolar macrophages in the early phase of acute pancreatitis. J Surg Res. 1996 Nov;66(1):43–50. doi: 10.1006/jsre.1996.0370. [DOI] [PubMed] [Google Scholar]
- Werner J., Rivera J., Fernandez-del Castillo C., Lewandrowski K., Adrie C., Rattner D. W., Warshaw A. L. Differing roles of nitric oxide in the pathogenesis of acute edematous versus necrotizing pancreatitis. Surgery. 1997 Jan;121(1):23–30. doi: 10.1016/s0039-6060(97)90178-1. [DOI] [PubMed] [Google Scholar]
- Werner J., Schmidt J., Warshaw A. L., Gebhard M. M., Herfarth C., Klar E. The relative safety of MRI contrast agent in acute necrotizing pancreatitis. Ann Surg. 1998 Jan;227(1):105–111. doi: 10.1097/00000658-199801000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Moncada S. Regulation of gastric mucosal integrity by endogenous nitric oxide: interactions with prostanoids and sensory neuropeptides in the rat. Br J Pharmacol. 1990 Mar;99(3):607–611. doi: 10.1111/j.1476-5381.1990.tb12977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn R. W., Currie M. G., Herman L. E. Nitric oxide participates in the regulation of pancreatic acinar cell secretion. Life Sci. 1994;55(7):511–518. doi: 10.1016/0024-3205(94)00743-8. [DOI] [PubMed] [Google Scholar]