Abstract
Background—During the interdigestive state in humans, erythromycin 40 mg induces a premature activity front that starts in the stomach, while erythromycin 200 mg induces a prolonged period of enhanced antral contractile activity. Aims—To study the involvement of a cholinergic pathway in the motor effects of erythromycin using the muscarinic antagonist atropine and the neural 5-HT1 receptor agonist sumatriptan. Methods—In 30 healthy volunteers, fasted antroduodenojejunal motor activity was studied by stationary manometry. Placebo (n=10), atropine (15 µg/kg intravenous bolus plus 15 µg/kg/h over 30 minutes; n=10), or sumatriptan (6 mg subcutaneously; n=10) was administered, followed by infusion of erythromycin 40 mg or 200mg. Results—After placebo, erythromycin 40 mg induced a premature activity front with gastric onset after 19.1 (1.7) minutes in all volunteers. After atropine, erythromycin 40 mg failed to induce a premature activity front during a 60 minute period in all volunteers (p<0.001), while sumatriptan prevented the induction of a premature activity front during a 60 minute period in all but one volunteer (p<0.005). The number of antral contractions and their mean amplitude in the 60 minutes after erythromycin 200 mg did not differ significantly after atropine or sumatriptan versus placebo. Conclusions—The antral motor effects of erythromycin in humans are mediated via different pathways. The induction of a premature activity front is mediated through activation of an intrinsic cholinergic pathway, while the induction of enhanced antral contractile activity may be mediated via a pathway potentially involving activation of a muscular receptor.
Keywords: migrating motor complex; cholinergic control; antral motility; atropine; sumatriptan
Full Text
The Full Text of this article is available as a PDF (145.9 KB).
Figure 1 .
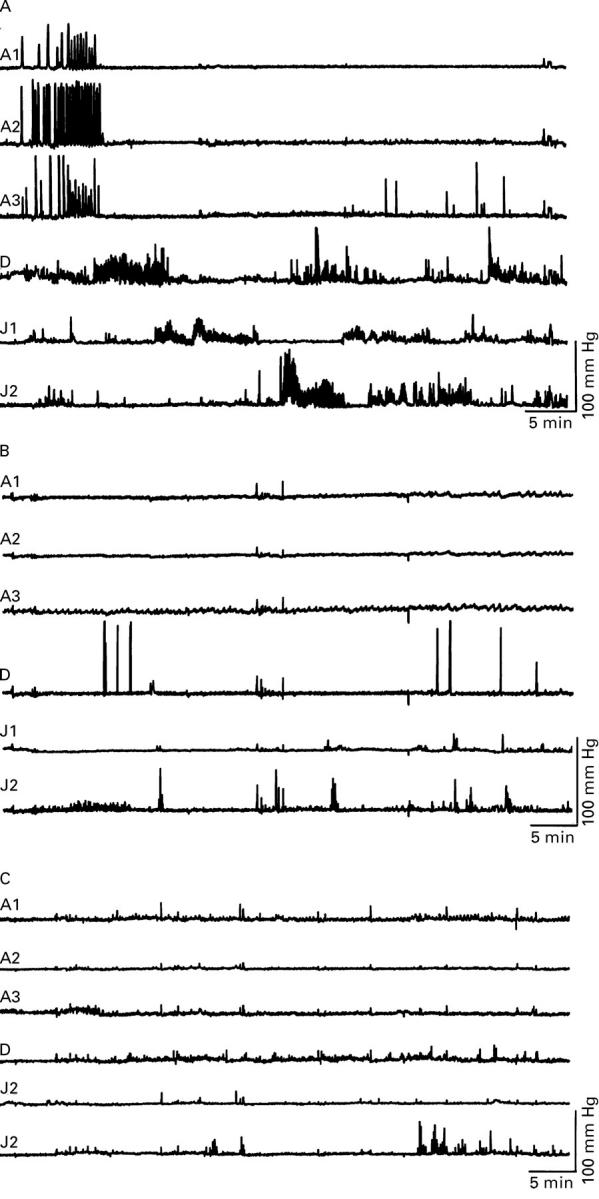
Effect of atropine and sumatriptan on the initiation of an antral activity front by erythromycin 40 mg intravenously in a healthy volunteer. All traces begin at the start of the infusion of erythromycin, which lasted 10 minutes. (A) Erythromycin caused a premature activity front at the antral level that migrated caudally to the small intestine. (B) Prior administration of atropine blocked the generation of a premature activity front by erythromycin during a 60 minute period. (C) Prior administration of sumatriptan also blocked the generation of a premature activity front by erythromycin during a 60 minute period.
Figure 2 .
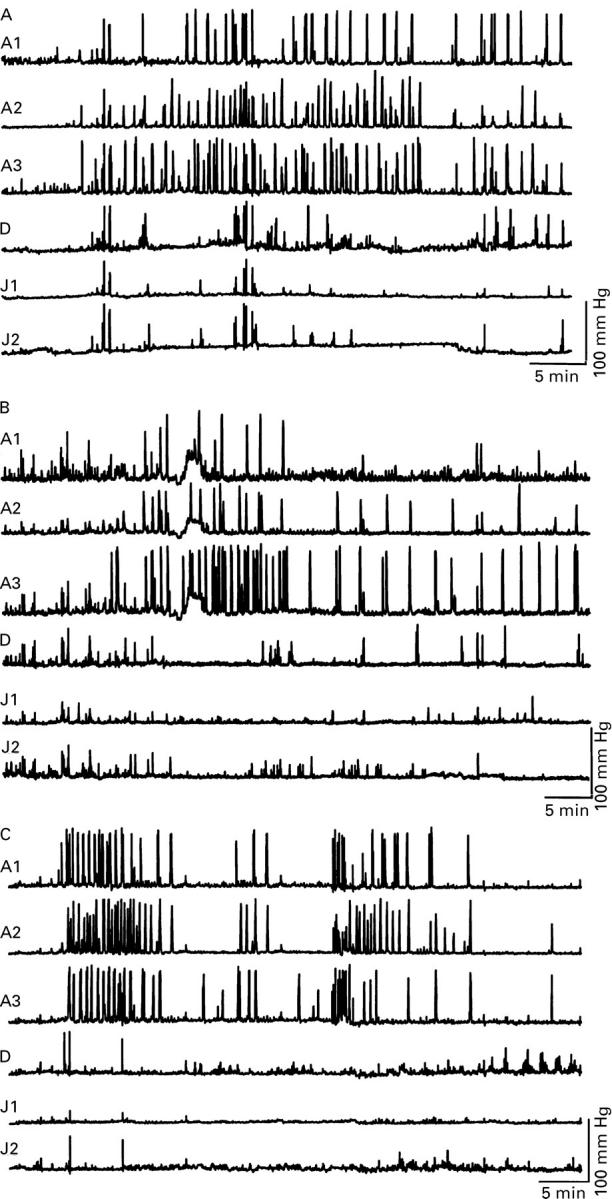
Effect of atropine and sumatriptan on antral contractile activity induced by erythromycin 200 mg intravenously in a healthy volunteer. All traces begin at the start of the infusion of erythromycin, which lasted 10 minutes. (A) Erythromycin caused a burst of prolonged contractile activity at the antral level that did not migrate to the small intestine and was not followed by a phase 1. (B) Prior administration of atropine did not influence the occurrence of this prolonged antral contractile activity. (C) Prior administration of sumatriptan also did not block the erythromycin induced prolonged antral contractile activity.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bormans V., Peeters T. L., Janssens J., Pearce D., Vandeweerd M., Vantrappen G. In man, only activity fronts that originate in the stomach correlate with motilin peaks. Scand J Gastroenterol. 1987 Sep;22(7):781–784. doi: 10.3109/00365528708991914. [DOI] [PubMed] [Google Scholar]
- Bormans V., Peeters T. L., Vantrappen G. Motilin receptors in rabbit stomach and small intestine. Regul Pept. 1986 Sep;15(2):143–153. doi: 10.1016/0167-0115(86)90084-4. [DOI] [PubMed] [Google Scholar]
- Chaussade S., Michopoulos S., Sogni P., Guerre J., Couturier D. Motilin agonist erythromycin increases human lower esophageal sphincter pressure by stimulation of cholinergic nerves. Dig Dis Sci. 1994 Feb;39(2):381–384. doi: 10.1007/BF02090212. [DOI] [PubMed] [Google Scholar]
- Coulie B., Tack J., Maes B., Geypens B., De Roo M., Janssens J. Sumatriptan, a selective 5-HT1 receptor agonist, induces a lag phase for gastric emptying of liquids in humans. Am J Physiol. 1997 Apr;272(4 Pt 1):G902–G908. doi: 10.1152/ajpgi.1997.272.4.G902. [DOI] [PubMed] [Google Scholar]
- Dechant K. L., Clissold S. P. Sumatriptan. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the acute treatment of migraine and cluster headache. Drugs. 1992 May;43(5):776–798. doi: 10.2165/00003495-199243050-00010. [DOI] [PubMed] [Google Scholar]
- Janssens J., Peeters T. L., Vantrappen G., Tack J., Urbain J. L., De Roo M., Muls E., Bouillon R. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med. 1990 Apr 12;322(15):1028–1031. doi: 10.1056/NEJM199004123221502. [DOI] [PubMed] [Google Scholar]
- Kuemmerle J. F., Kellum J. M. Serotonin neural receptors mediate motilin-induced motility in isolated, vascularly perfused canine jejunum. J Surg Res. 1988 Oct;45(4):357–362. doi: 10.1016/0022-4804(88)90131-x. [DOI] [PubMed] [Google Scholar]
- Lüdtke F. E., Müller H., Golenhofen K. Direct effects of motilin on isolated smooth muscle from various regions of the human stomach. Pflugers Arch. 1989 Sep;414(5):558–563. doi: 10.1007/BF00580991. [DOI] [PubMed] [Google Scholar]
- Mawe G. M., Branchek T. A., Gershon M. D. Blockade of 5-HT-mediated enteric slow EPSPs by BRL 24924: gastrokinetic effects. Am J Physiol. 1989 Sep;257(3 Pt 1):G386–G396. doi: 10.1152/ajpgi.1989.257.3.G386. [DOI] [PubMed] [Google Scholar]
- Parkman H. P., Pagano A. P., Vozzelli M. A., Ryan J. P. Gastrokinetic effects of erythromycin: myogenic and neurogenic mechanisms of action in rabbit stomach. Am J Physiol. 1995 Sep;269(3 Pt 1):G418–G426. doi: 10.1152/ajpgi.1995.269.3.G418. [DOI] [PubMed] [Google Scholar]
- Peeters T. L., Bormans V., Vantrappen G. Comparison of motilin binding to crude homogenates of human and canine gastrointestinal smooth muscle tissue. Regul Pept. 1988 Nov;23(2):171–182. doi: 10.1016/0167-0115(88)90025-0. [DOI] [PubMed] [Google Scholar]
- Peeters T. L. Erythromycin and other macrolides as prokinetic agents. Gastroenterology. 1993 Dec;105(6):1886–1899. doi: 10.1016/0016-5085(93)91089-z. [DOI] [PubMed] [Google Scholar]
- Peeters T., Matthijs G., Depoortere I., Cachet T., Hoogmartens J., Vantrappen G. Erythromycin is a motilin receptor agonist. Am J Physiol. 1989 Sep;257(3 Pt 1):G470–G474. doi: 10.1152/ajpgi.1989.257.3.G470. [DOI] [PubMed] [Google Scholar]
- Poitras P., Lahaie R. G., St-Pierre S., Trudel L. Comparative stimulation of motilin duodenal receptor by porcine or canine motilin. Gastroenterology. 1987 Mar;92(3):658–662. doi: 10.1016/0016-5085(87)90014-x. [DOI] [PubMed] [Google Scholar]
- Poitras P., Miller P., Dickner M., Mao Y. K., Daniel E. E., St-Pierre S., Trudel L. Heterogeneity of motilin receptors in the gastrointestinal tract of the rabbit. Peptides. 1996;17(4):701–707. doi: 10.1016/0196-9781(96)00053-8. [DOI] [PubMed] [Google Scholar]
- Satoh M., Sakai T., Sano I., Fujikura K., Koyama H., Ohshima K., Itoh Z., Omura S. EM574, an erythromycin derivative, is a potent motilin receptor agonist in human gastric antrum. J Pharmacol Exp Ther. 1994 Oct;271(1):574–579. [PubMed] [Google Scholar]
- Tack J., Janssens J., Vantrappen G., Peeters T., Annese V., Depoortere I., Muls E., Bouillon R. Effect of erythromycin on gastric motility in controls and in diabetic gastroparesis. Gastroenterology. 1992 Jul;103(1):72–79. doi: 10.1016/0016-5085(92)91097-n. [DOI] [PubMed] [Google Scholar]
- Urbain J. L., Vantrappen G., Janssens J., Van Cutsem E., Peeters T., De Roo M. Intravenous erythromycin dramatically accelerates gastric emptying in gastroparesis diabeticorum and normals and abolishes the emptying discrimination between solids and liquids. J Nucl Med. 1990 Sep;31(9):1490–1493. [PubMed] [Google Scholar]
- Vantrappen G., Janssens J., Hellemans J., Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977 Jun;59(6):1158–1166. doi: 10.1172/JCI108740. [DOI] [PMC free article] [PubMed] [Google Scholar]


