Abstract
Background—It has been proposed that a pathogenic effect of Helicobacter pylori is a weakening of the protective mucus barrier; however, this remains controversial. Aims—To clarify the effects of H pylori infection on the mucus gel barrier in vivo. Methods—Mucus gel polymeric structure and the thickness of the adherent mucus barrier were measured in endoscopic biopsy samples in subjects with and without H pylori infection. Results—There was a significant 18% reduction in the proportion of polymeric gel forming mucin in the adherent mucus layer in H pylori positive compared with negative subjects. There was no change in the adherent mucus thickness between H pylori positive and negative subjects without gastric atrophy (mean (SD): 104(26) µm, 106 (30) µm respectively). There was however a significant reduction in mucus thickness in those H pylori positive subjects with underlying gastric atrophy (84 (13) µm, p=0.03) compared with those without atrophy. Conclusions—A partial breakdown in gel forming structure of the gastric mucus barrier does occur in H pylori infection per se but this is insufficient to cause a collapse of the mucus barrier.
Keywords: Helicobacter pylori; gastric mucus
Full Text
The Full Text of this article is available as a PDF (122.3 KB).
Figure 1 .
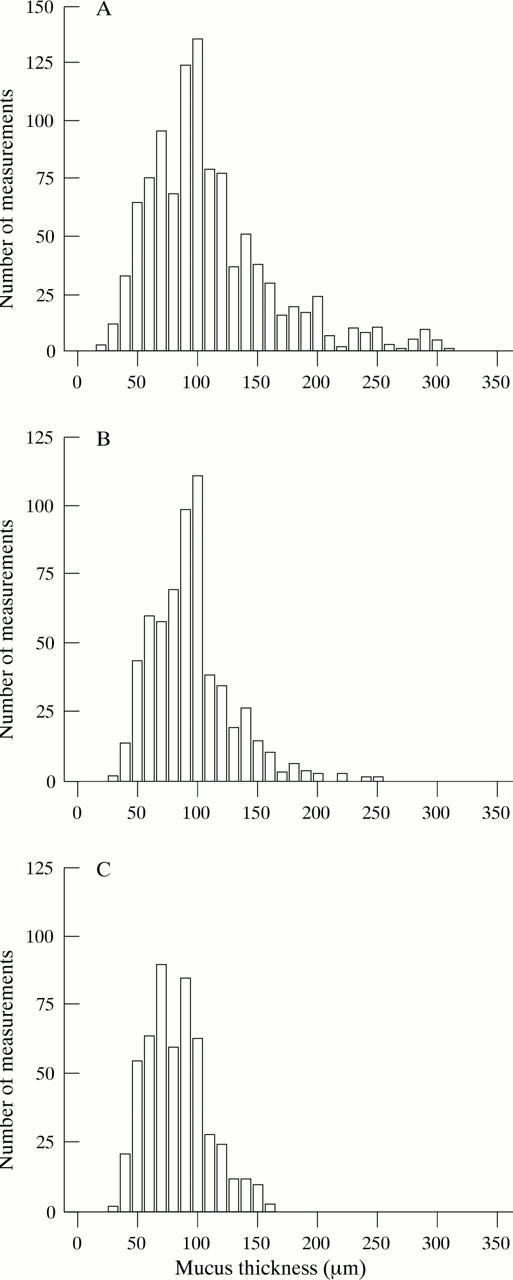
Individual mucus thickness measurements (measurements within ±5 µm) shown according to H pylori status and the presence or absence of underlying gastric atrophy. (A) H pylori negative; (B) H pylori positive without atrophy; (C) H pylori positive with atrophy.
Figure 2 .
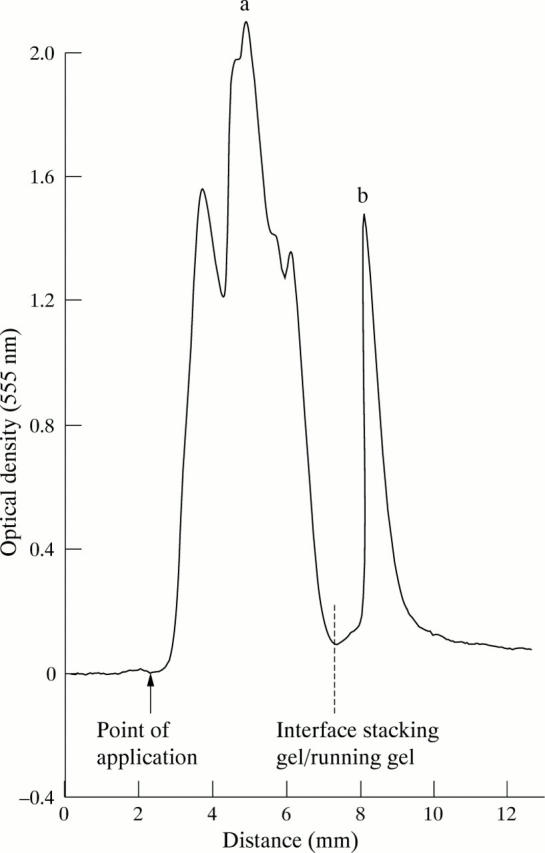
Representative SDS-PAGE of gastric antral mucin showing 2 distinct bands. Band a: polymeric large molecular weight mucin that enters the stacking gel; mucin applied to gel enters the stacking gel and diffuses around the point of application as one broad band. Band b: smaller sized mucin that enters the running gel; dotted line shows the interface between the stacking and the running gel.
Figure 3 .
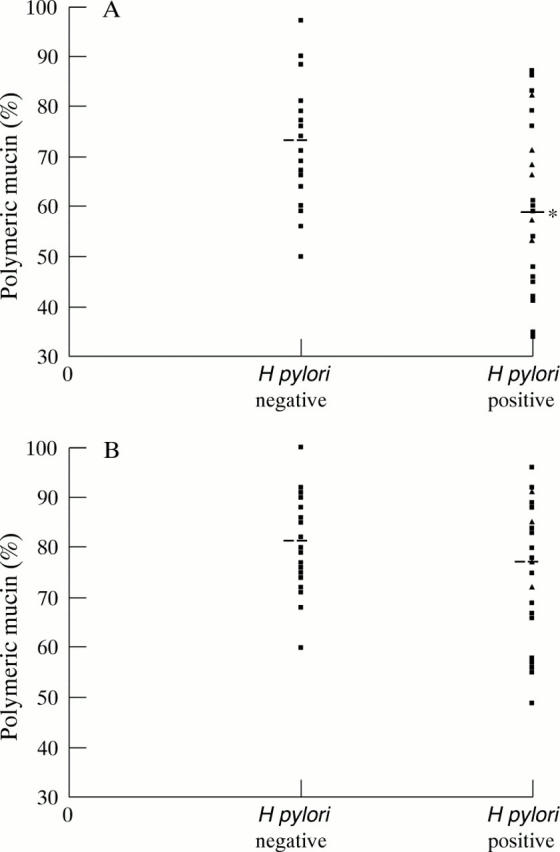
Percentage ratio of large molecular weight polymeric mucin in: (A) mucosal surface brushings (adherent mucus gel layer); and (B) biopsy samples (intracellular mucus and adherent mucus gel layer). Dotted lines represent mean values. *p=0.01.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Flemström G., Garner A., Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev. 1993 Oct;73(4):823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- Baxter A., Campbell C. J., Cox D. M., Grinham C. J., Pendlebury J. E. Proteolytic activities of human Campylobacter pylori and ferret gastric Campylobacter-like organism. Biochem Biophys Res Commun. 1989 Aug 30;163(1):1–7. doi: 10.1016/0006-291x(89)92089-5. [DOI] [PubMed] [Google Scholar]
- Byrd J. C., Yan P., Sternberg L., Yunker C. K., Scheiman J. M., Bresalier R. S. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997 Aug;113(2):455–464. doi: 10.1053/gast.1997.v113.pm9247464. [DOI] [PubMed] [Google Scholar]
- Hutton D. A., Pearson J. P., Allen A., Foster S. N. Mucolysis of the colonic mucus barrier by faecal proteinases: inhibition by interacting polyacrylate. Clin Sci (Lond) 1990 Mar;78(3):265–271. doi: 10.1042/cs0780265. [DOI] [PubMed] [Google Scholar]
- Kerss S., Allen A., Garner A. A simple method for measuring thickness of the mucus gel layer adherent to rat, frog and human gastric mucosa: influence of feeding, prostaglandin, N-acetylcysteine and other agents. Clin Sci (Lond) 1982 Aug;63(2):187–195. doi: 10.1042/cs0630187. [DOI] [PubMed] [Google Scholar]
- Kuipers E. J., Lee A., Klinkenberg-Knol E. C., Meuwissen S. G. Review article: the development of atrophic gastritis--Helicobacter pylori and the effects of acid suppressive therapy. Aliment Pharmacol Ther. 1995 Aug;9(4):331–340. doi: 10.1111/j.1365-2036.1995.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Mantle M., Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings]. Biochem Soc Trans. 1978;6(3):607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- Markesich D. C., Anand B. S., Lew G. M., Graham D. Y. Helicobacter pylori infection does not reduce the viscosity of human gastric mucus gel. Gut. 1995 Mar;36(3):327–329. doi: 10.1136/gut.36.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. J., Barrett L. J., Prakash C., McCallum R. W., Guerrant R. L. Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology. 1990 Sep;99(3):697–702. doi: 10.1016/0016-5085(90)90957-3. [DOI] [PubMed] [Google Scholar]
- Marshall B. J. Helicobacter pylori. Am J Gastroenterol. 1994 Aug;89(8 Suppl):S116–S128. [PubMed] [Google Scholar]
- Ota H., Katsuyama T. Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem J. 1992 Feb;24(2):86–92. doi: 10.1007/BF01082444. [DOI] [PubMed] [Google Scholar]
- Pearson J., Allen A., Venables C. Gastric mucus: isolation and polymeric structure of the undegraded glycoprotein: its breakdown by pepsin. Gastroenterology. 1980 Apr;78(4):709–715. [PubMed] [Google Scholar]
- Rankin B. J., Srivastava E. D., Record C. O., Pearson J. P., Allen A. Patients with ulcerative colitis have reduced mucin polymer content in the adherent colonic mucus gel. Biochem Soc Trans. 1995 Feb;23(1):104S–104S. doi: 10.1042/bst023104s. [DOI] [PubMed] [Google Scholar]
- Sarosiek J., Marshall B. J., Peura D. A., Hoffman S., Feng T., McCallum R. W. Gastroduodenal mucus gel thickness in patients with Helicobacter pylori: a method for assessment of biopsy specimens. Am J Gastroenterol. 1991 Jun;86(6):729–734. [PubMed] [Google Scholar]
- Sarosiek J., Slomiany A., Slomiany B. L. Evidence for weakening of gastric mucus integrity by Campylobacter pylori. Scand J Gastroenterol. 1988 Jun;23(5):585–590. doi: 10.3109/00365528809093916. [DOI] [PubMed] [Google Scholar]
- Schade C., Flemström G., Holm L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology. 1994 Jul;107(1):180–188. doi: 10.1016/0016-5085(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Sellers L. A., Allen A., Morris E. R., Ross-Murphy S. B. Mucus glycoprotein gels. Role of glycoprotein polymeric structure and carbohydrate side-chains in gel-formation. Carbohydr Res. 1988 Jul 15;178:93–110. doi: 10.1016/0008-6215(88)80104-6. [DOI] [PubMed] [Google Scholar]
- Sidebotham R. L., Batten J. J., Karim Q. N., Spencer J., Baron J. H. Breakdown of gastric mucus in presence of Helicobacter pylori. J Clin Pathol. 1991 Jan;44(1):52–57. doi: 10.1136/jcp.44.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany B. L., Bilski J., Sarosiek J., Murty V. L., Dworkin B., VanHorn K., Zielenski J., Slomiany A. Campylobacter pyloridis degrades mucin and undermines gastric mucosal integrity. Biochem Biophys Res Commun. 1987 Apr 14;144(1):307–314. doi: 10.1016/s0006-291x(87)80511-9. [DOI] [PubMed] [Google Scholar]
- Thomsen L. L., Gavin J. B., Tasman-Jones C. Relation of Helicobacter pylori to the human gastric mucosa in chronic gastritis of the antrum. Gut. 1990 Nov;31(11):1230–1236. doi: 10.1136/gut.31.11.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van-Seuningen I., Davril M. A rapid periodic acid-Schiff staining procedure for the detection of glycoproteins using the PhastSystem. Electrophoresis. 1992 Jan-Feb;13(1-2):97–99. doi: 10.1002/elps.1150130119. [DOI] [PubMed] [Google Scholar]
- Younan F., Pearson J., Allen A., Venables C. Changes in the structure of the mucous gel on the mucosal surface of the stomach in association with peptic ulcer disease. Gastroenterology. 1982 May;82(5 Pt 1):827–831. [PubMed] [Google Scholar]


