Abstract
Laboratory of Molecular Therapy,Imperial Cancer Research Fund Molecular Oncology Unit,Imperial College School of Medicine,Hammersmith Hospital, London W12 0NN, UK
H PANDHA, N R LEMOINE
Gene therapy, in particular the transfer of genes encoding immunostimulatory molecules (cytokines and costimulatory molecules) as well as selectively cytotoxic enzymes and DNA vaccination, has the potential of enhancing cell mediated immune responses against tumours including those of colorectal origin. Genes can be transferred using viral vectors either to cultured tumour cells in vitro that can be returned to the patient as a "cancer vaccine", or directly to tumour cells in vivo. Vaccination with DNA constructs expressing specific tumour antigens characteristic of colorectal neoplasia can trigger immune recognition and destruction of tumour cells. The aim is to tip the balance from protumour to antitumour mechanisms by generating a local immune response and systemic antitumour immune memory to destroy metastases. Studies in murine models, combined with human studies, show that such approaches could become an adjunct to current treatments for human colorectal cancer in the near future.
Full Text
The Full Text of this article is available as a PDF (127.3 KB).
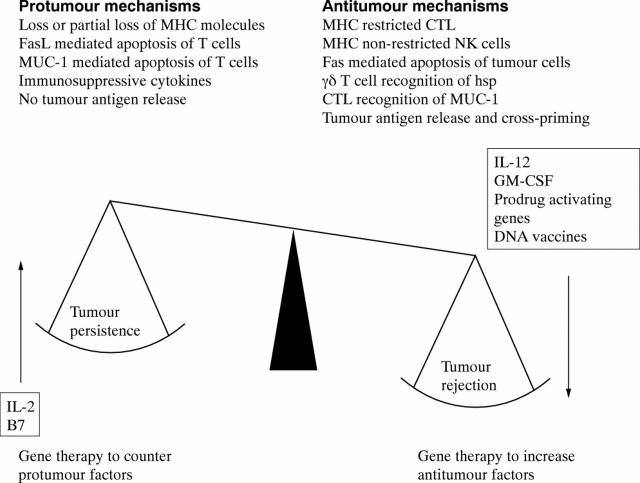
Figure 1 .
Tipping the balance in colorectal cancer. A number of protumour mechanisms outweigh antitumour mechanisms and allow colorectal tumours to survive and proliferate. Gene therapy with cytokine, immunostimulatory or suicide (prodrug activating) gene transfer to tumour cells, or DNA vaccination, aims to tip the balance towards antitumour mechanisms and tumour rejection by enhancing antitumour cellular immune responses. Correction of immune deficiencies associated with the tumour could work in synergy with enhancement of antitumour immunity and many of the genes shown could apply to both sides of the balance.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acres R. B., Hareuveni M., Balloul J. M., Kieny M. P. Vaccinia virus MUC1 immunization of mice: immune response and protection against the growth of murine tumors bearing the MUC1 antigen. J Immunother Emphasis Tumor Immunol. 1993 Aug;14(2):136–143. [PubMed] [Google Scholar]
- Allione A., Consalvo M., Nanni P., Lollini P. L., Cavallo F., Giovarelli M., Forni M., Gulino A., Colombo M. P., Dellabona P. Immunizing and curative potential of replicating and nonreplicating murine mammary adenocarcinoma cells engineered with interleukin (IL)-2, IL-4, IL-6, IL-7, IL-10, tumor necrosis factor alpha, granulocyte-macrophage colony-stimulating factor, and gamma-interferon gene or admixed with conventional adjuvants. Cancer Res. 1994 Dec 1;54(23):6022–6026. [PubMed] [Google Scholar]
- Avery A., Paraskeva C., Hall P., Flanders K. C., Sporn M., Moorghen M. TGF-beta expression in the human colon: differential immunostaining along crypt epithelium. Br J Cancer. 1993 Jul;68(1):137–139. doi: 10.1038/bjc.1993.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C. M., Riley L. B., Bae Y. J., Salmeron M. A., Platsoucas C. D., von Eschenbach A., Itoh K. Patterns of human tumor-infiltrating lymphocytes in 120 human cancers. Arch Surg. 1990 Feb;125(2):200–205. doi: 10.1001/archsurg.1990.01410140078012. [DOI] [PubMed] [Google Scholar]
- Barnd D. L., Lan M. S., Metzgar R. S., Finn O. J. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman W. J., Donnellan I., Fraser I. A., Wong L. S., Morris A. G. Lymphocytes infiltrating colorectal cancer have low proliferative capacity but can secrete normal levels of interferon gamma. Cancer Immunol Immunother. 1995 Jul;41(1):61–67. doi: 10.1007/BF01788961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M., Petronzelli F., Bicknell D., Krausa P., Rowan A., Tonks S., Murray N., Bodmer J., Bodmer W. Mechanisms of loss of HLA class I expression on colorectal tumor cells. Tissue Antigens. 1996 May;47(5):364–371. doi: 10.1111/j.1399-0039.1996.tb02571.x. [DOI] [PubMed] [Google Scholar]
- Burg C., Patry Y., Le Pendu J., Moreau M., Tesson L., Godard A., Soulillou J. P., Meflah K., Anegon I. Leukaemia Inhibitory Factor derived from rat colon carcinoma cells increases host susceptibility to tumour growth. Cytokine. 1995 Nov;7(8):784–792. doi: 10.1006/cyto.1995.0094. [DOI] [PubMed] [Google Scholar]
- Caruso M., Pham-Nguyen K., Kwong Y. L., Xu B., Kosai K. I., Finegold M., Woo S. L., Chen S. H. Adenovirus-mediated interleukin-12 gene therapy for metastatic colon carcinoma. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11302–11306. doi: 10.1073/pnas.93.21.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. H., Kosai K., Xu B., Pham-Nguyen K., Contant C., Finegold M. J., Woo S. L. Combination suicide and cytokine gene therapy for hepatic metastases of colon carcinoma: sustained antitumor immunity prolongs animal survival. Cancer Res. 1996 Aug 15;56(16):3758–3762. [PubMed] [Google Scholar]
- Chong H., Todryk S., Hutchinson G., Hart I. R., Vile R. G. Tumour cell expression of B7 costimulatory molecules and interleukin-12 or granulocyte-macrophage colony-stimulating factor induces a local antitumour response and may generate systemic protective immunity. Gene Ther. 1998 Feb;5(2):223–232. doi: 10.1038/sj.gt.3300584. [DOI] [PubMed] [Google Scholar]
- Colombo M. P., Vagliani M., Spreafico F., Parenza M., Chiodoni C., Melani C., Stoppacciaro A. Amount of interleukin 12 available at the tumor site is critical for tumor regression. Cancer Res. 1996 Jun 1;56(11):2531–2534. [PubMed] [Google Scholar]
- Conry R. M., LoBuglio A. F., Loechel F., Moore S. E., Sumerel L. A., Barlow D. L., Curiel D. T. A carcinoembryonic antigen polynucleotide vaccine has in vivo antitumor activity. Gene Ther. 1995 Jan;2(1):59–65. [PubMed] [Google Scholar]
- Corr M., Lee D. J., Carson D. A., Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996 Oct 1;184(4):1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit D., Flemming C. L., Harris J. D., Palmer K. J., Moore J. S., Gore M. E., Collins M. K. IL-12 stimulation but not B7 expression increases melanoma killing by patient cytotoxic T lymphocytes (CTL). Clin Exp Immunol. 1996 Aug;105(2):353–359. doi: 10.1046/j.1365-2249.1996.d01-773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio A., Botti C., Tocchi A., Mingazzini P., Flammia M. The influence of tumor lymphocytic infiltration on long term survival of surgically treated colorectal cancer patients. Int Surg. 1992 Oct-Dec;77(4):256–260. [PubMed] [Google Scholar]
- Diaz R. M., Todryk S., Chong H., Hart I. R., Sikora K., Dorudi S., Vile R. G. Rapid adenoviral transduction of freshly resected tumour explants with therapeutically useful genes provides a rationale for genetic immunotherapy for colorectal cancer. Gene Ther. 1998 Jul;5(7):869–879. doi: 10.1038/sj.gt.3300690. [DOI] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Finn O. J., Jerome K. R., Henderson R. A., Pecher G., Domenech N., Magarian-Blander J., Barratt-Boyes S. M. MUC-1 epithelial tumor mucin-based immunity and cancer vaccines. Immunol Rev. 1995 Jun;145:61–89. doi: 10.1111/j.1600-065x.1995.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Fossum B., Gedde-Dahl T., 3rd, Hansen T., Eriksen J. A., Thorsby E., Gaudernack G. Overlapping epitopes encompassing a point mutation (12 Gly-->Arg) in p21 ras can be recognized by HLA-DR, -DP and -DQ restricted T cells. Eur J Immunol. 1993 Oct;23(10):2687–2691. doi: 10.1002/eji.1830231045. [DOI] [PubMed] [Google Scholar]
- Fossum B., Olsen A. C., Thorsby E., Gaudernack G. CD8+ T cells from a patient with colon carcinoma, specific for a mutant p21-Ras-derived peptide (Gly13-->Asp), are cytotoxic towards a carcinoma cell line harbouring the same mutation. Cancer Immunol Immunother. 1995 Mar;40(3):165–172. doi: 10.1007/BF01517348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastl G. A., Abrams J. S., Nanus D. M., Oosterkamp R., Silver J., Liu F., Chen M., Albino A. P., Bander N. H. Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer. 1993 Aug 19;55(1):96–101. doi: 10.1002/ijc.2910550118. [DOI] [PubMed] [Google Scholar]
- Gimmi C. D., Morrison B. W., Mainprice B. A., Gribben J. G., Boussiotis V. A., Freeman G. J., Park S. Y., Watanabe M., Gong J., Hayes D. F. Breast cancer-associated antigen, DF3/MUC1, induces apoptosis of activated human T cells. Nat Med. 1996 Dec;2(12):1367–1370. doi: 10.1038/nm1296-1367. [DOI] [PubMed] [Google Scholar]
- Graham R. A., Burchell J. M., Beverley P., Taylor-Papadimitriou J. Intramuscular immunisation with MUC1 cDNA can protect C57 mice challenged with MUC1-expressing syngeneic mouse tumour cells. Int J Cancer. 1996 Mar 1;65(5):664–670. doi: 10.1002/(SICI)1097-0215(19960301)65:5<664::AID-IJC17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gunji Y., Tagawa M., Matsubara H., Takenaga K., Shimada H., Kondo F., Suzuki T., Nakajima K., Sugaya M., Asano T. Antitumor effect induced by the expression of granulocyte macrophage-colony stimulating factor gene in murine colon carcinoma cells. Cancer Lett. 1996 Mar 29;101(2):257–261. doi: 10.1016/0304-3835(96)04141-9. [DOI] [PubMed] [Google Scholar]
- Harada M., Matsunaga K., Oguchi Y., Iijima H., Ito O., Tamada K., Kimura G., Nomoto K. The involvement of transforming growth factor beta in the impaired antitumor T-cell response at the gut-associated lymphoid tissue (GALT). Cancer Res. 1995 Dec 15;55(24):6146–6151. [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Henderson R. A., Nimgaonkar M. T., Watkins S. C., Robbins P. D., Ball E. D., Finn O. J. Human dendritic cells genetically engineered to express high levels of the human epithelial tumor antigen mucin (MUC-1). Cancer Res. 1996 Aug 15;56(16):3763–3770. [PubMed] [Google Scholar]
- Hom S. S., Rosenberg S. A., Topalian S. L. Specific immune recognition of autologous tumor by lymphocytes infiltrating colon carcinomas: analysis by cytokine secretion. Cancer Immunol Immunother. 1993;36(1):1–8. doi: 10.1007/BF01789124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover H. C., Jr, Brandhorst J. S., Peters L. C., Surdyke M. G., Takeshita Y., Madariaga J., Muenz L. R., Hanna M. G., Jr Adjuvant active specific immunotherapy for human colorectal cancer: 6.5-year median follow-up of a phase III prospectively randomized trial. J Clin Oncol. 1993 Mar;11(3):390–399. doi: 10.1200/JCO.1993.11.3.390. [DOI] [PubMed] [Google Scholar]
- Huang A. Y., Golumbek P., Ahmadzadeh M., Jaffee E., Pardoll D., Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994 May 13;264(5161):961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- Hwu P., Yannelli J., Kriegler M., Anderson W. F., Perez C., Chiang Y., Schwarz S., Cowherd R., Delgado C., Mulé J. Functional and molecular characterization of tumor-infiltrating lymphocytes transduced with tumor necrosis factor-alpha cDNA for the gene therapy of cancer in humans. J Immunol. 1993 May 1;150(9):4104–4115. [PubMed] [Google Scholar]
- Jackson P. A., Green M. A., Marks C. G., King R. J., Hubbard R., Cook M. G. Lymphocyte subset infiltration patterns and HLA antigen status in colorectal carcinomas and adenomas. Gut. 1996 Jan;38(1):85–89. doi: 10.1136/gut.38.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob L., Somasundaram R., Smith W., Monos D., Basak S., Marincola F., Pereira S., Herlyn D. Cytotoxic T-cell clone against rectal carcinoma induced by stimulation of a patient's peripheral blood mononuclear cells with autologous cultured tumor cells. Int J Cancer. 1997 May 2;71(3):325–332. doi: 10.1002/(sici)1097-0215(19970502)71:3<325::aid-ijc3>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kaklamanis L., Townsend A., Doussis-Anagnostopoulou I. A., Mortensen N., Harris A. L., Gatter K. C. Loss of major histocompatibility complex-encoded transporter associated with antigen presentation (TAP) in colorectal cancer. Am J Pathol. 1994 Sep;145(3):505–509. [PMC free article] [PubMed] [Google Scholar]
- Kantor J., Irvine K., Abrams S., Kaufman H., DiPietro J., Schlom J. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. J Natl Cancer Inst. 1992 Jul 15;84(14):1084–1091. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- Kim J. A., Martin E. W., Jr, Morgan C. J., Aldrich W., Triozzi P. L. Expansion of mucin-reactive T-helper lymphocytes from patients with colorectal cancer. Cancer Biother. 1995 Summer;10(2):115–123. doi: 10.1089/cbr.1995.10.115. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996 Oct 18;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Sunouchi K., Ono M., Sawada T., Muto T. Local immunity and metastasis of colorectal carcinoma. Dis Colon Rectum. 1992 Jul;35(7):645–650. doi: 10.1007/BF02053754. [DOI] [PubMed] [Google Scholar]
- Lazaris A. C., Theodoropoulos G. E., Davaris P. S., Panoussopoulos D., Nakopoulou L., Kittas C., Golematis B. C. Heat shock protein 70 and HLA-DR molecules tissue expression. Prognostic implications in colorectal cancer. Dis Colon Rectum. 1995 Jul;38(7):739–745. doi: 10.1007/BF02048033. [DOI] [PubMed] [Google Scholar]
- Levitsky H. I., Lazenby A., Hayashi R. J., Pardoll D. M. In vivo priming of two distinct antitumor effector populations: the role of MHC class I expression. J Exp Med. 1994 Apr 1;179(4):1215–1224. doi: 10.1084/jem.179.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Berencsi K., Basak S., Somasundaram R., Ricciardi R. P., Gönczöl E., Zaloudik J., Linnenbach A., Maruyama H., Miniou P. Human colorectal cancer (CRC) antigen CO17-1A/GA733 encoded by adenovirus inhibits growth of established CRC cells in mice. J Immunol. 1997 Jul 15;159(2):763–769. [PubMed] [Google Scholar]
- Lindauer M., Schackert H. K., Gebert J., Rudy W., Habicht A., Siebels M., Meuer S. C., Moebius U. Immune reactions induced by interleukin-2 transfected colorectal cancer cells in vitro: predominant induction of lymphokine-activated killer cells. J Mol Med (Berl) 1996 Jan;74(1):43–49. doi: 10.1007/BF00202071. [DOI] [PubMed] [Google Scholar]
- Maeurer M. J., Martin D., Walter W., Liu K., Zitvogel L., Halusczcak K., Rabinowich H., Duquesnoy R., Storkus W., Lotze M. T. Human intestinal Vdelta1+ lymphocytes recognize tumor cells of epithelial origin. J Exp Med. 1996 Apr 1;183(4):1681–1696. doi: 10.1084/jem.183.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeurer M. J., Walter W., Martin D., Zitvogel L., Elder E., Storkus W., Lotze M. T. Interleukin-7 (IL-7) in colorectal cancer: IL-7 is produced by tissues from colorectal cancer and promotes preferential expansion of tumour infiltrating lymphocytes. Scand J Immunol. 1997 Feb;45(2):182–192. doi: 10.1046/j.1365-3083.1997.d01-384.x. [DOI] [PubMed] [Google Scholar]
- Martinotti A., Stoppacciaro A., Vagliani M., Melani C., Spreafico F., Wysocka M., Parmiani G., Trinchieri G., Colombo M. P. CD4 T cells inhibit in vivo the CD8-mediated immune response against murine colon carcinoma cells transduced with interleukin-12 genes. Eur J Immunol. 1995 Jan;25(1):137–146. doi: 10.1002/eji.1830250124. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Petersson M., Lenkei R., Taupin J. L., Magnusson I., Mellstedt H., Anderson P., Kiessling R. Alterations in the signal-transducing molecules of T cells and NK cells in colorectal tumor-infiltrating, gut mucosal and peripheral lymphocytes: correlation with the stage of the disease. Int J Cancer. 1995 Jun 9;61(6):765–772. doi: 10.1002/ijc.2910610605. [DOI] [PubMed] [Google Scholar]
- Melcher A., Todryk S., Hardwick N., Ford M., Jacobson M., Vile R. G. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med. 1998 May;4(5):581–587. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- Mori M., Inoue H., Mimori K., Shibuta K., Baba K., Nakashima H., Haraguchi M., Tsuji K., Ueo H., Barnard G. F. Expression of MAGE genes in human colorectal carcinoma. Ann Surg. 1996 Aug;224(2):183–188. doi: 10.1097/00000658-199608000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G., Botzler C., Jennen L., Schmidt J., Ellwart J., Issels R. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol. 1997 May 1;158(9):4341–4350. [PubMed] [Google Scholar]
- Möller P., Koretz K., Leithäuser F., Brüderlein S., Henne C., Quentmeier A., Krammer P. H. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer. 1994 May 1;57(3):371–377. doi: 10.1002/ijc.2910570314. [DOI] [PubMed] [Google Scholar]
- Nabel G. J., Gordon D., Bishop D. K., Nickoloff B. J., Yang Z. Y., Aruga A., Cameron M. J., Nabel E. G., Chang A. E. Immune response in human melanoma after transfer of an allogeneic class I major histocompatibility complex gene with DNA-liposome complexes. Proc Natl Acad Sci U S A. 1996 Dec 24;93(26):15388–15393. doi: 10.1073/pnas.93.26.15388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi H., Petersson M., Magnusson I., Juhlin C., Matsuda M., Mellstedt H., Taupin J. L., Vivier E., Anderson P., Kiessling R. Decreased expression of the signal-transducing zeta chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 1993 Dec 1;53(23):5610–5612. [PubMed] [Google Scholar]
- Nanda N. K., Sercarz E. E. Induction of anti-self-immunity to cure cancer. Cell. 1995 Jul 14;82(1):13–17. doi: 10.1016/0092-8674(95)90047-0. [DOI] [PubMed] [Google Scholar]
- O'Connell J., O'Sullivan G. C., Collins J. K., Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996 Sep 1;184(3):1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohwada A., Hirschowitz E. A., Crystal R. G. Regional delivery of an adenovirus vector containing the Escherichia coli cytosine deaminase gene to provide local activation of 5-fluorocytosine to suppress the growth of colon carcinoma metastatic to liver. Hum Gene Ther. 1996 Aug 20;7(13):1567–1576. doi: 10.1089/hum.1996.7.13-1567. [DOI] [PubMed] [Google Scholar]
- Ostenstad B., Lea T., Schlichting E., Harboe M. Human colorectal tumour infiltrating lymphocytes express activation markers and the CD45RO molecule, showing a primed population of lymphocytes in the tumour area. Gut. 1994 Mar;35(3):382–387. doi: 10.1136/gut.35.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenstad B., Sioud M., Lea T., Schlichting E., Harboe M. Limited heterogeneity in the T-cell receptor V-gene usage in lymphocytes infiltrating human colorectal tumours. Br J Cancer. 1994 Jun;69(6):1078–1082. doi: 10.1038/bjc.1994.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H., Chen W., Takahashi M., Disis M. L., Byrd D. R., McCahill L., Bertram K. A., Fenton R. G., Peace D. J., Cheever M. A. CD4+ T-cell immunity to mutated ras protein in pancreatic and colon cancer patients. Cancer Res. 1995 Jul 15;55(14):2984–2987. [PubMed] [Google Scholar]
- Rajasekar R., Sim G. K., Augustin A. Self heat shock and gamma delta T-cell reactivity. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1767–1771. doi: 10.1073/pnas.87.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom J. H., Pelle B. A., Hubers H., Keynton L. M., Hanna M. G., Jr, Pomato N. Identification of colon-tumor-associated antigens by T-cell lines derived from tumor-infiltrating lymphocytes and peripheral-blood lymphocytes from patients immunized with an autologous tumor-cell/bacillus Calmette-Guérin vaccine. Int J Cancer. 1993 Jul 9;54(5):734–740. doi: 10.1002/ijc.2910540505. [DOI] [PubMed] [Google Scholar]
- Reeves M. E., Royal R. E., Lam J. S., Rosenberg S. A., Hwu P. Retroviral transduction of human dendritic cells with a tumor-associated antigen gene. Cancer Res. 1996 Dec 15;56(24):5672–5677. [PubMed] [Google Scholar]
- Rodolfo M., Zilocchi C., Melani C., Cappetti B., Arioli I., Parmiani G., Colombo M. P. Immunotherapy of experimental metastases by vaccination with interleukin gene-transduced adenocarcinoma cells sharing tumor-associated antigens. Comparison between IL-12 and IL-2 gene-transduced tumor cell vaccines. J Immunol. 1996 Dec 15;157(12):5536–5542. [PubMed] [Google Scholar]
- Rosenberg S. A. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997 Apr;18(4):175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- Roth J. A., Cristiano R. J. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997 Jan 1;89(1):21–39. doi: 10.1093/jnci/89.1.21. [DOI] [PubMed] [Google Scholar]
- Rubin J., Galanis E., Pitot H. C., Richardson R. L., Burch P. A., Charboneau J. W., Reading C. C., Lewis B. D., Stahl S., Akporiaye E. T. Phase I study of immunotherapy of hepatic metastases of colorectal carcinoma by direct gene transfer of an allogeneic histocompatibility antigen, HLA-B7. Gene Ther. 1997 May;4(5):419–425. doi: 10.1038/sj.gt.3300396. [DOI] [PubMed] [Google Scholar]
- Sensi M., Parmiani G. Analysis of TCR usage in human tumors: a new tool for assessing tumor-specific immune responses. Immunol Today. 1995 Dec;16(12):588–595. doi: 10.1016/0167-5699(95)80082-4. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Iwatsuki S., Herberman R. B., Whiteside T. L. Effects of cytokines on in vitro growth of tumor-infiltrating lymphocytes obtained from human primary and metastatic liver tumors. Cancer Immunol Immunother. 1991;32(5):280–288. doi: 10.1007/BF01789045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. E., Marsh S. G., Bodmer J. G., Gelsthorpe K., Bodmer W. F. Loss of HLA-A,B,C allele products and lymphocyte function-associated antigen 3 in colorectal neoplasia. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5557–5561. doi: 10.1073/pnas.86.14.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennevig J. L., Lunde O. C., Holter J., Bjørgsvik D. Lymphoid infiltration and prognosis in colorectal carcinoma. Br J Cancer. 1984 Mar;49(3):375–377. doi: 10.1038/bjc.1984.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takii Y., Hashimoto S., Iiai T., Watanabe H., Hatakeyama K., Abo T. Increase in the proportion of granulated CD56+ T cells in patients with malignancy. Clin Exp Immunol. 1994 Sep;97(3):522–527. doi: 10.1111/j.1365-2249.1994.tb06120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Kanai F., Okabe S., Yoshida Y., Wakimoto H., Hamada H., Shiratori Y., Lan K., Ishitobi M., Omata M. Adenovirus-mediated prodrug gene therapy for carcinoembryonic antigen-producing human gastric carcinoma cells in vitro. Cancer Res. 1996 Mar 15;56(6):1341–1345. [PubMed] [Google Scholar]
- Tepper R. I., Mulé J. J. Experimental and clinical studies of cytokine gene-modified tumor cells. Hum Gene Ther. 1994 Feb;5(2):153–164. doi: 10.1089/hum.1994.5.2-153. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Rogers M., Edwards J., Mukherjee S., Bastin J. Source of unique tumour antigens. Nature. 1994 Oct 20;371(6499):662–662. doi: 10.1038/371662a0. [DOI] [PubMed] [Google Scholar]
- Trinh Q. T., Austin E. A., Murray D. M., Knick V. C., Huber B. E. Enzyme/prodrug gene therapy: comparison of cytosine deaminase/5-fluorocytosine versus thymidine kinase/ganciclovir enzyme/prodrug systems in a human colorectal carcinoma cell line. Cancer Res. 1995 Nov 1;55(21):4808–4812. [PubMed] [Google Scholar]
- Tsang K. Y., Zaremba S., Nieroda C. A., Zhu M. Z., Hamilton J. M., Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995 Jul 5;87(13):982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- Tsushima H., Kawata S., Tamura S., Ito N., Shirai Y., Kiso S., Imai Y., Shimomukai H., Nomura Y., Matsuda Y. High levels of transforming growth factor beta 1 in patients with colorectal cancer: association with disease progression. Gastroenterology. 1996 Feb;110(2):375–382. doi: 10.1053/gast.1996.v110.pm8566583. [DOI] [PubMed] [Google Scholar]
- Vile R. G., Castleden S., Marshall J., Camplejohn R., Upton C., Chong H. Generation of an anti-tumour immune response in a non-immunogenic tumour: HSVtk killing in vivo stimulates a mononuclear cell infiltrate and a Th1-like profile of intratumoural cytokine expression. Int J Cancer. 1997 Apr 10;71(2):267–274. doi: 10.1002/(sici)1097-0215(19970410)71:2<267::aid-ijc23>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Vile R. G., Nelson J. A., Castleden S., Chong H., Hart I. R. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994 Dec 1;54(23):6228–6234. [PubMed] [Google Scholar]
- Vile R., Russell S. J. Gene transfer technologies for the gene therapy of cancer. Gene Ther. 1994 Mar;1(2):88–98. [PubMed] [Google Scholar]
- Watanabe N., Hizuta A., Tanaka N., Orita K. Localization of T cell receptor (TCR)-gamma delta + T cells into human colorectal cancer: flow cytometric analysis of TCR-gamma delta expression in tumour-infiltrating lymphocytes. Clin Exp Immunol. 1995 Oct;102(1):167–173. doi: 10.1111/j.1365-2249.1995.tb06651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Zhao X., Kariya Y., Fukata H., Teshigawara K., Uchida A. Induction of autologous tumor killing by heat treatment of fresh human tumor cells: involvement of gamma delta T cells and heat shock protein 70. Cancer Res. 1996 Mar 1;56(5):1104–1110. [PubMed] [Google Scholar]
- Yanuck M., Carbone D. P., Pendleton C. D., Tsukui T., Winter S. F., Minna J. D., Berzofsky J. A. A mutant p53 tumor suppressor protein is a target for peptide-induced CD8+ cytotoxic T-cells. Cancer Res. 1993 Jul 15;53(14):3257–3261. [PubMed] [Google Scholar]
- Yoo Y. K., Heo D. S., Hata K., Van Thiel D. H., Whiteside T. L. Tumor-infiltrating lymphocytes from human colon carcinomas. Functional and phenotypic characteristics after long-term culture in recombinant interleukin 2. Gastroenterology. 1990 Feb;98(2):259–268. [PubMed] [Google Scholar]
- Zier K., Gansbacher B., Salvadori S. Preventing abnormalities in signal transduction of T cells in cancer: the promise of cytokine gene therapy. Immunol Today. 1996 Jan;17(1):39–45. doi: 10.1016/0167-5699(96)80567-6. [DOI] [PubMed] [Google Scholar]
- van de Wiel-van Kemenade E., Ligtenberg M. J., de Boer A. J., Buijs F., Vos H. L., Melief C. J., Hilkens J., Figdor C. G. Episialin (MUC1) inhibits cytotoxic lymphocyte-target cell interaction. J Immunol. 1993 Jul 15;151(2):767–776. [PubMed] [Google Scholar]