Abstract
Background—Hyperbaric oxygen (HBO) has been suggested to be beneficial in inflammatory bowel disease but the mechanisms responsible for its therapeutic effects have not been elucidated. Aim—To assess the effect of HBO treatment on colonic damage in two models of experimental colitis, and to examine whether this effect is mediated by modulation of NO synthesis. Methods—Colitis was induced by either flushing the colon with 2 ml 5% acetic acid or intracolonic administration of 30 mg trinitrobenzenesulphonic acid (TNB) dissolved in 0.25 ml 50% ethanol. Rats were exposed to HBO (100% oxygen at 2.4 atmosphere absolute) for one hour twice on the day of colitis induction and once daily thereafter. Control rats were treated only with acetic acid or TNB. Rats were killed 24 hours after acetic acid administration or one and seven days after TNB treatment. The colon was isolated, washed, and weighed, the lesion area was measured, and mucosal scrapings were processed for determination of myeloperoxidase (MPO) and NO synthase (NOS) activities, prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) generation. Results—In control rats exposed for seven days to HBO, colonic NOS activity was significantly decreased by 61%, compared with its activity in untreated rats (2.93 (0.17) nmol/g/min). HBO significantly reduced by 51 and 62% the extent of injury induced by acetic acid and TNB respectively. The protection provided by HBO was accompanied by a significant decrease in colonic weight, PGE2 generation, MPO, and NOS activities. In acetic acid colitis, LTB4 generation was also significantly decreased. Conclusions—(1) HBO effectively decreases colitis induced by acetic acid and TNB. (2) The decreased NOS activity induced by HBO suggests that reduction in NO generation may be among the mechanisms responsible for the anti-inflammatory effect of HBO. (3) HBO may be considered in the treatment of patients with refractory inflammatory bowel disease.
Keywords: hyperbaric oxygen; acetic acid colitis; trinitrobenzenesulphonic acid colitis; inflammatory bowel disease
Full Text
The Full Text of this article is available as a PDF (173.6 KB).
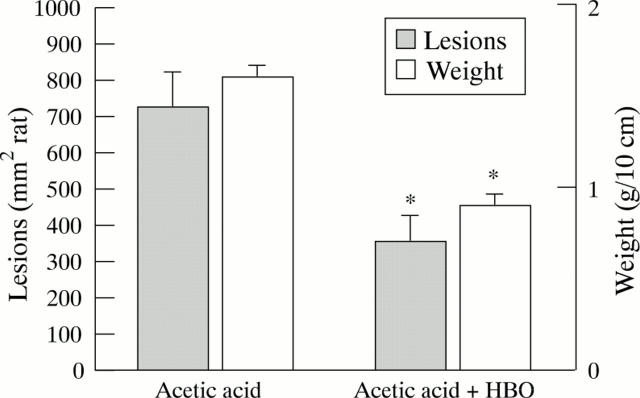
Figure 1 .
Effect of hyperbaric oxygen (HBO) on colonic lesion area and weight in acetic acid treated rats. Colitis was induced by injection of 2.0 ml 5% acetic acid into the proximal colon. One group of rats was exposed twice for one hour to HBO (100% oxygen at 2.4 ATA). Rats were killed after 24 hours. The colon was isolated and weighed and the lesion area measured. Results are mean (SEM) for 8-16 rats in each group. *Significantly different from acetic acid only (p<0.05).
Figure 2 .
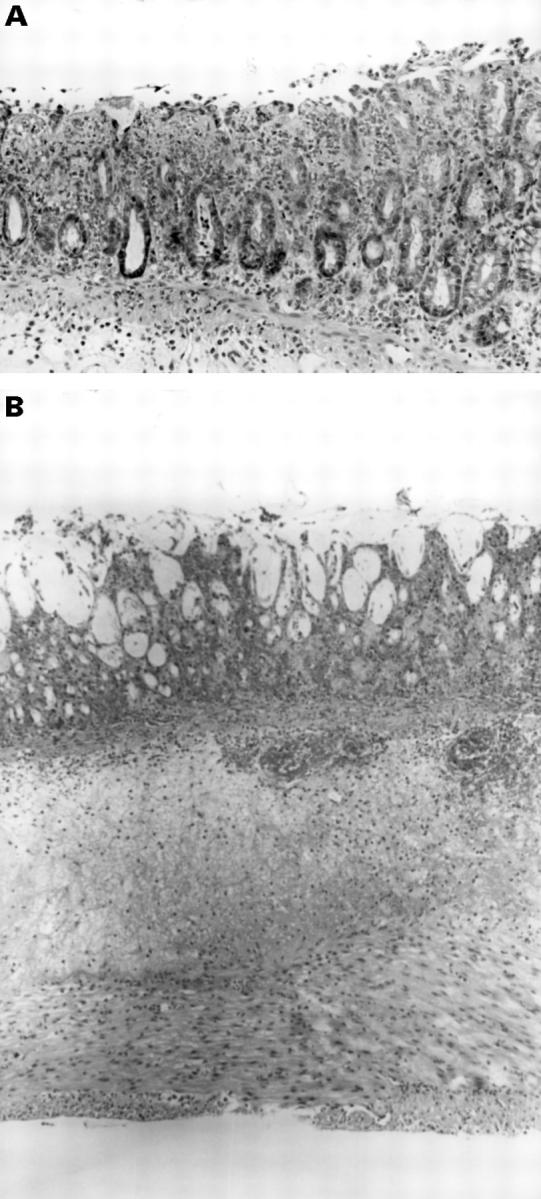
Histological section of the colon isolated from rats treated with acetic acid and hyperbaric oxygen. (A) Small mucosal ulcerations can be seen with an acute mild inflammatory cell infiltrate involving the upper third to one half of the mucosal thickness. Haematoxylin and eosin staining; original magnification × 187. (B) Wide mucosal ulceration can be seen with an extensive inflammatory cell infiltrate involving all layers of the intestinal wall. Haematoxylin and eosin staining; original magnification × 71.
Figure 3 .
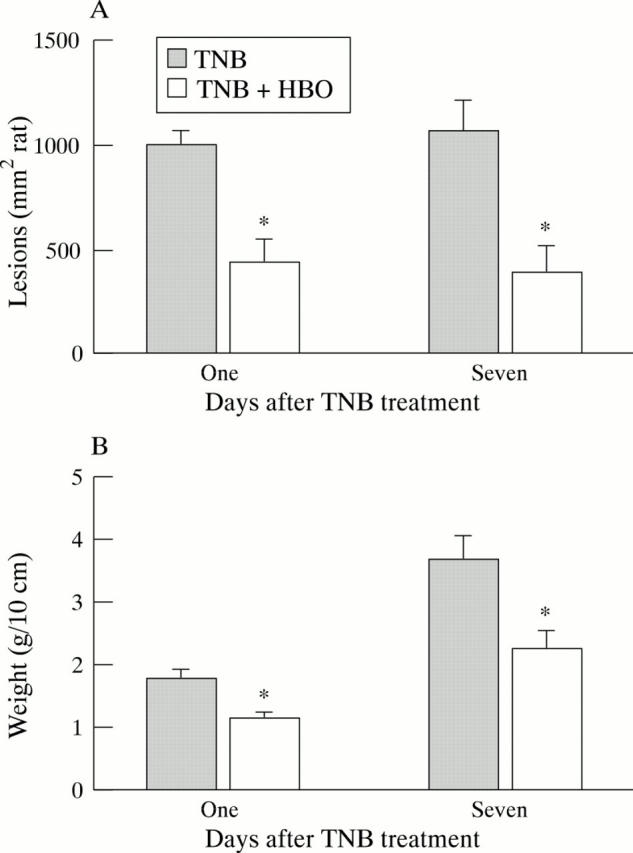
Effect of hyperbaric oxygen (HBO) on colonic lesion area (A) and weight (B) in rats treated with trinitrobenzenesulphonic acid (TNB). Colitis was induced by intracolonic administration of TNB. Rats were treated for one hour with HBO (100% oxygen at 2.4 ATA) twice daily in the first 24 hours and once daily thereafter. They were killed after one or seven days. The distal 10 cm of the colon was isolated and weighed and the lesion area measured. Results are mean (SEM) for 8-12 rats in each group. *Significantly different from TNB alone (p<0.05).
Figure 4 .
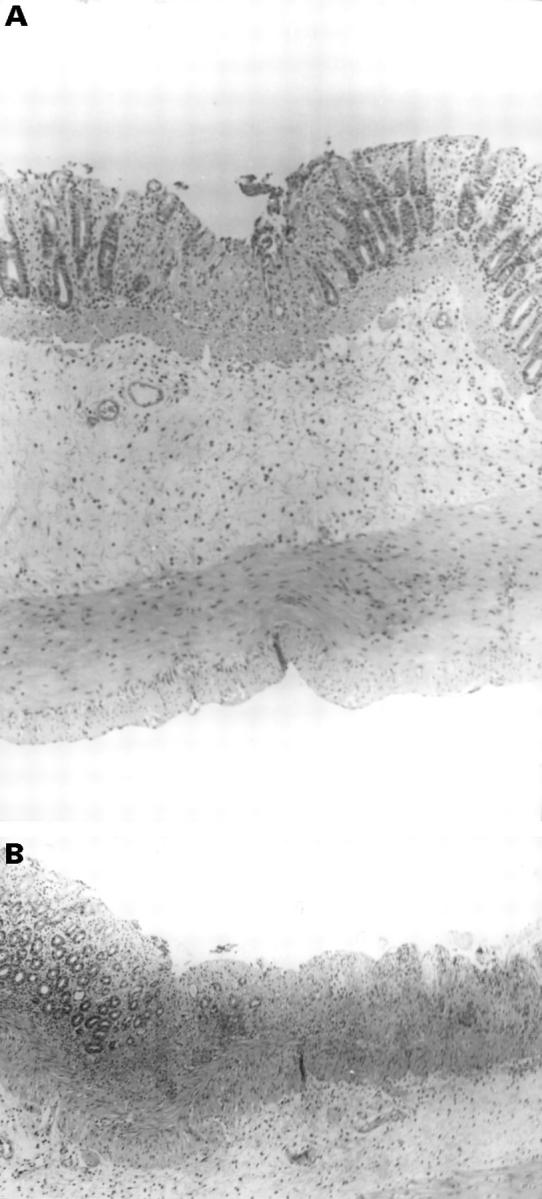
Histological section of the colon isolated 24 hours after intracolonic treatment with trinitrobenzenesulphonic acid and exposure to hyperbaric oxygen. (A) A small superficial ulcer can be seen involving only the mucosa and accompanied by a very mild acute inflammatory cell infiltrate. Haematoxylin and eosin staining; original magnification × 71. (B) Wide and extensive mucosal ulcers are evident. Haematoxylin and eosin staining; original magnification × 71.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982 Mar;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Bush P. A., Gonzalez N. E., Griscavage J. M., Ignarro L. J. Nitric oxide synthase from cerebellum catalyzes the formation of equimolar quantities of nitric oxide and citrulline from L-arginine. Biochem Biophys Res Commun. 1992 Jun 30;185(3):960–966. doi: 10.1016/0006-291x(92)91720-b. [DOI] [PubMed] [Google Scholar]
- Colombel J. F., Mathieu D., Bouault J. M., Lesage X., Zavadil P., Quandalle P., Cortot A. Hyperbaric oxygenation in severe perineal Crohn's disease. Dis Colon Rectum. 1995 Jun;38(6):609–614. doi: 10.1007/BF02054120. [DOI] [PubMed] [Google Scholar]
- Gabb G., Robin E. D. Hyperbaric oxygen. A therapy in search of diseases. Chest. 1987 Dec;92(6):1074–1082. doi: 10.1378/chest.92.6.1074. [DOI] [PubMed] [Google Scholar]
- Karmeli F., Eliakim R., Okon E., Samuni A., Rachmilewitz D. A stable nitroxide radical effectively decreases mucosal damage in experimental colitis. Gut. 1995 Sep;37(3):386–393. doi: 10.1136/gut.37.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata S., Yamashita U., Nakajima H. Hyperbaric oxygenation reduces the cytostatic activity and transcription of nitric oxide synthetase gene of mouse peritoneal macrophages. Biochim Biophys Acta. 1995 Jul 25;1263(1):35–38. doi: 10.1016/0167-4781(95)00084-t. [DOI] [PubMed] [Google Scholar]
- Lavy A., Weisz G., Adir Y., Ramon Y., Melamed Y., Eidelman S. Hyperbaric oxygen for perianal Crohn's disease. J Clin Gastroenterol. 1994 Oct;19(3):202–205. doi: 10.1097/00004836-199410000-00006. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Karmeli F., Okon E., Bursztyn M. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 1995 Aug;37(2):247–255. doi: 10.1136/gut.37.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz D., Karmeli F., Okon E. Sulfhydryl blocker-induced rat colonic inflammation is ameliorated by inhibition of nitric oxide synthase. Gastroenterology. 1995 Jul;109(1):98–106. doi: 10.1016/0016-5085(95)90273-2. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Simon P. L., Schwartz L. W., Griswold D. E., Fondacaro J. D., Wasserman M. A. Inflammatory mediators of experimental colitis in rats. Gastroenterology. 1989 Aug;97(2):326–337. doi: 10.1016/0016-5085(89)90068-1. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Stamler J. S., Bachwich D., Karmeli F., Ackerman Z., Podolsky D. K. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut. 1995 May;36(5):718–723. doi: 10.1136/gut.36.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz D., Stamler J. S., Karmeli F., Mullins M. E., Singel D. J., Loscalzo J., Xavier R. J., Podolsky D. K. Peroxynitrite-induced rat colitis--a new model of colonic inflammation. Gastroenterology. 1993 Dec;105(6):1681–1688. doi: 10.1016/0016-5085(93)91063-n. [DOI] [PubMed] [Google Scholar]
- Robert A., Nezamis J. E., Lancaster C., Hanchar A. J. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979 Sep;77(3):433–443. [PubMed] [Google Scholar]
- Sharon P., Ligumsky M., Rachmilewitz D., Zor U. Role of prostaglandins in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine. Gastroenterology. 1978 Oct;75(4):638–640. [PubMed] [Google Scholar]
- Tibbles P. M., Edelsberg J. S. Hyperbaric-oxygen therapy. N Engl J Med. 1996 Jun 20;334(25):1642–1648. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- Yamada T., Taguchi T., Hirata Y., Suita S., Yagi H. The protective effect of hyperbaric oxygenation on the small intestine in ischemia-reperfusion injury. J Pediatr Surg. 1995 Jun;30(6):786–790. doi: 10.1016/0022-3468(95)90748-3. [DOI] [PubMed] [Google Scholar]
- Zamboni W. A., Roth A. C., Russell R. C., Graham B., Suchy H., Kucan J. O. Morphologic analysis of the microcirculation during reperfusion of ischemic skeletal muscle and the effect of hyperbaric oxygen. Plast Reconstr Surg. 1993 May;91(6):1110–1123. doi: 10.1097/00006534-199305000-00022. [DOI] [PubMed] [Google Scholar]