Abstract
Background—Overproduction of nitric oxide (NO) via induction of the inducible NO synthase (iNOS) is an important factor in the haemodynamic disturbances of several inflammatory states. Aims—To identify the role of NO in a caerulein induced model of acute pancreatitis in the rat. Methods—Arterial blood pressure and plasma NO metabolites were measured at zero and seven hours in adult male Wistar rats administered caerulein (n=10) or saline (n=10). Pancreatic activity of NOS (inducible and constitutive) was assayed biochemically. The pancreatic expression and cellular localisation of NOS and nitrotyrosine (a marker of peroxynitrite induced oxidative tissue damage) were characterised immunohistochemically. Results—Compared with controls at seven hours, the pancreatitis group displayed raised plasma NO metabolites (mean (SEM) 70.2(5.9) versus 22.7 (2.2) µmol/l, p<0.0001) and reduced mean arterial blood pressure (88.7 (4.6) versus 112.8 (4.1) mm Hg, p=0.008). There was notable iNOS activity in the pancreatitis group (3.1(0.34) versus 0.1 (0.01) pmol/mg protein/min, p<0.0001) with reduced constitutive NOS activity (0.62 (0.12) versus 0.96 (0.08) pmol/mg protein/min, p=0.031). The increased expression of iNOS was mainly localised within vascular smooth muscle cells (p=0.003 versus controls), with positive perivascular staining for nitrotyrosine (p=0.0012 versus controls). Conclusions—In this experimental model of acute pancreatitis, iNOS induction and oxidative tissue damage in the pancreas is associated with raised systemic NO and arterial hypotension. Excess production of NO arising from the inducible NO synthase may be an important factor in the systemic and local haemodynamic disturbances associated with acute pancreatitis.
Keywords: acute pancreatitis; nitric oxide; inducible nitric oxide synthase; peroxynitrite; caerulein induced pancreatitis
Full Text
The Full Text of this article is available as a PDF (240.0 KB).
Figure 1 .
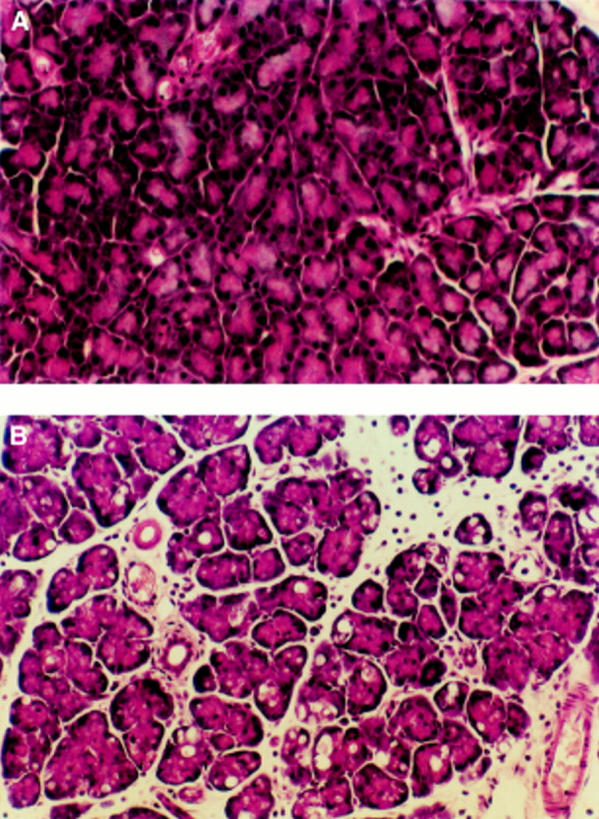
(A) Microscopic section of the pancreas from the control group, with normal appearance of acini. (B) Microscopic section of the pancreas from the pancreatitis group, showing the features of acute oedematous pancreatitis notably interstitial oedema, intracytoplasmic vacuoles, and extensive infiltration of the section with inflammatory cells (original magnification ×100).
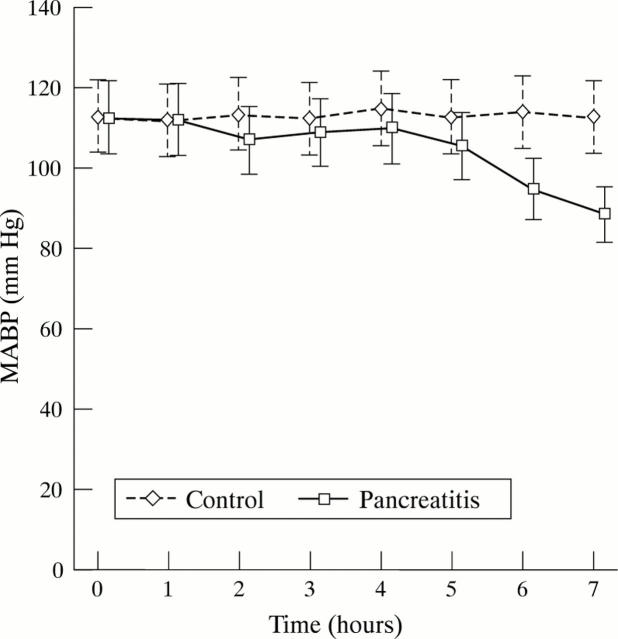
Figure 2 .
Mean (95% CI) arterial blood pressure (MABP) throughout the procedure in the control and the pancreatitis groups. p<0.0001 for the interaction between group and time using split plot repeated measures ANOVA. There was a significant difference between the control group and the pancreatitis group at six hours (p=0.046) and seven hours (p=0.008) only (using Bonferroni correction).
Figure 3 .
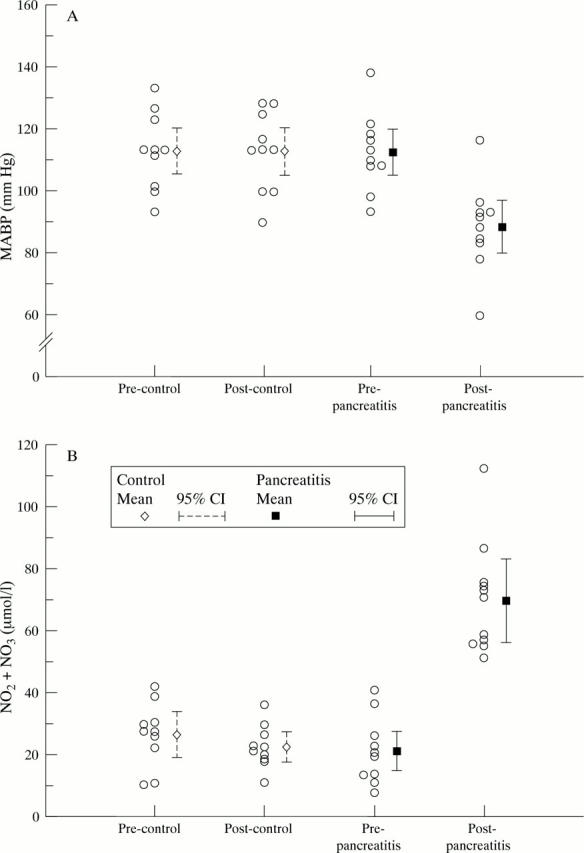
(A) Mean arterial blood pressure (MABP) measurement at the start and end of the procedure (zero and seven hours). (B) Plasma NO metabolites concentrations at the start and end of the procedure in control and pancreatitis groups.
Figure 4 .
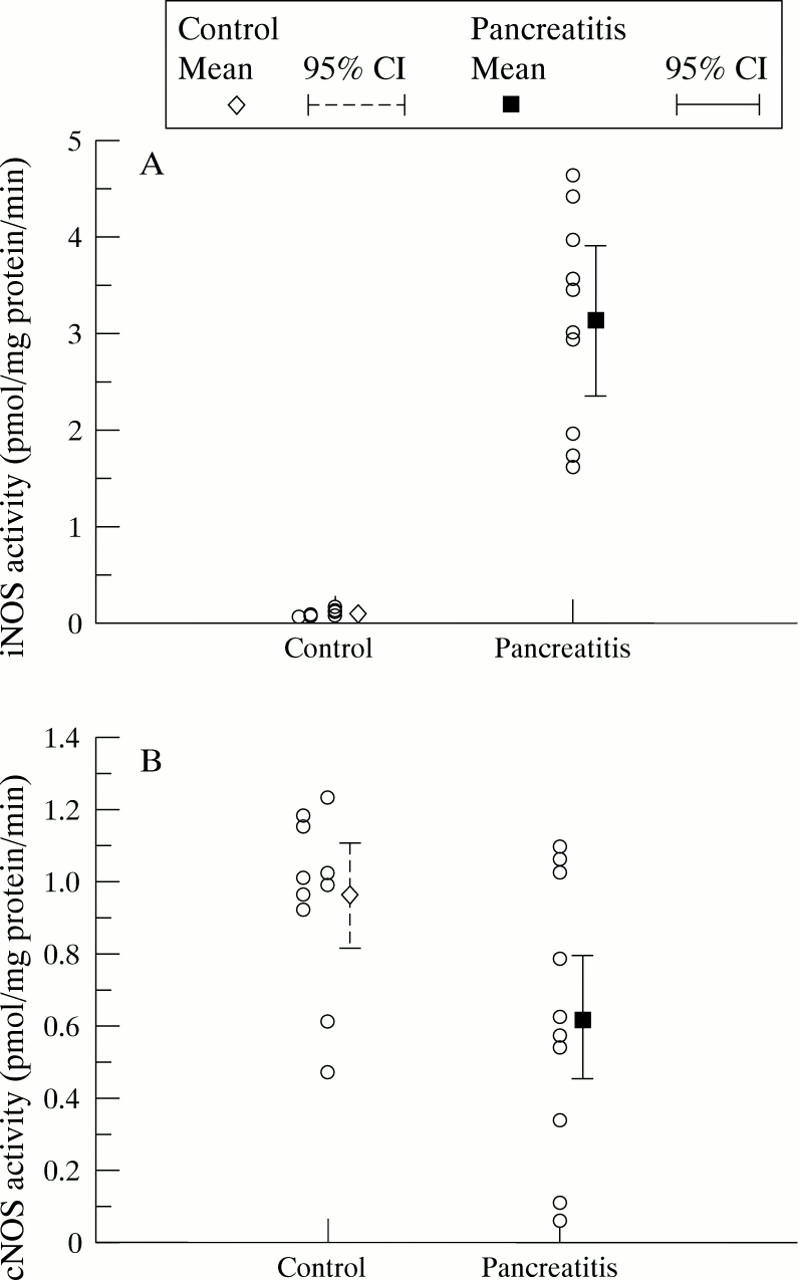
Biochemical assay of NOS activity. (A) iNOS activity (p<0.0001); (B) cNOS activity (p=0.031).
Figure 5 .
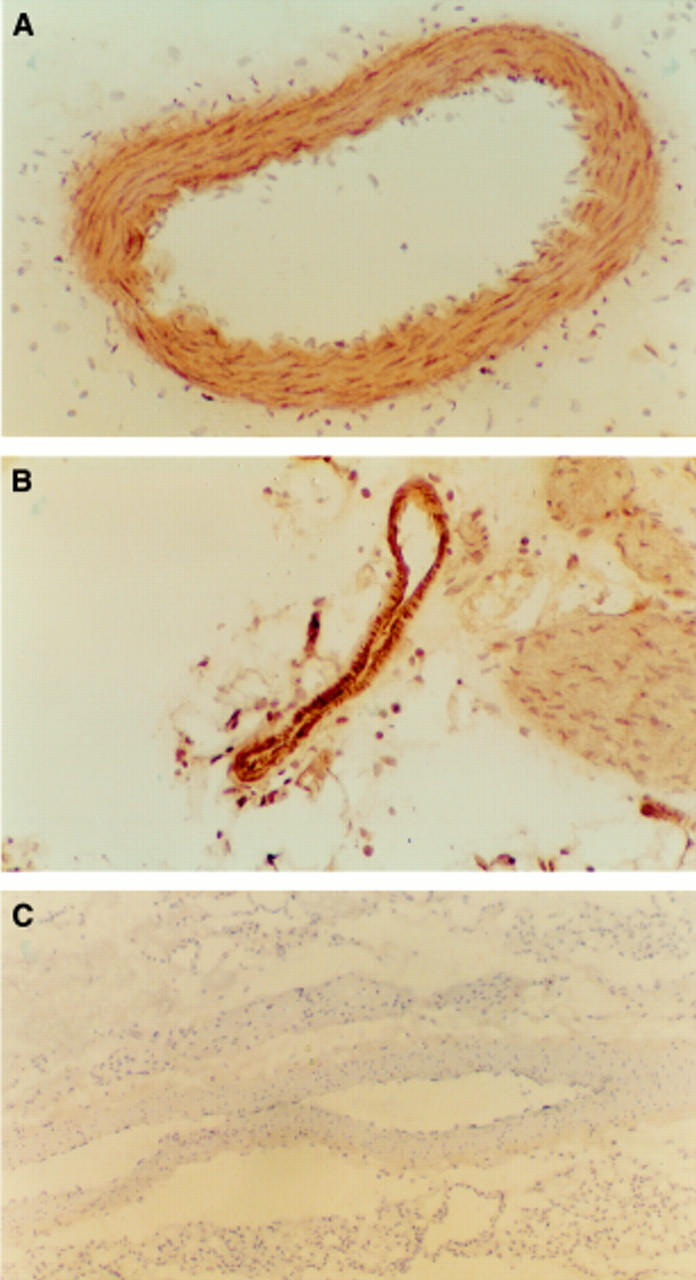
(A) Section from the pancreatitis group with positive staining for iNOS of the vascular smooth muscle cells. (B) Section from one of the three rats from the pancreatitis group with positive staining of the endothelial cells with the anti-iNOS antibody. (C) Section from the control group with negative staining of the vascular smooth muscle and endothelial cells with the anti-iNOS antibody (original magnification ×200).
Figure 6 .
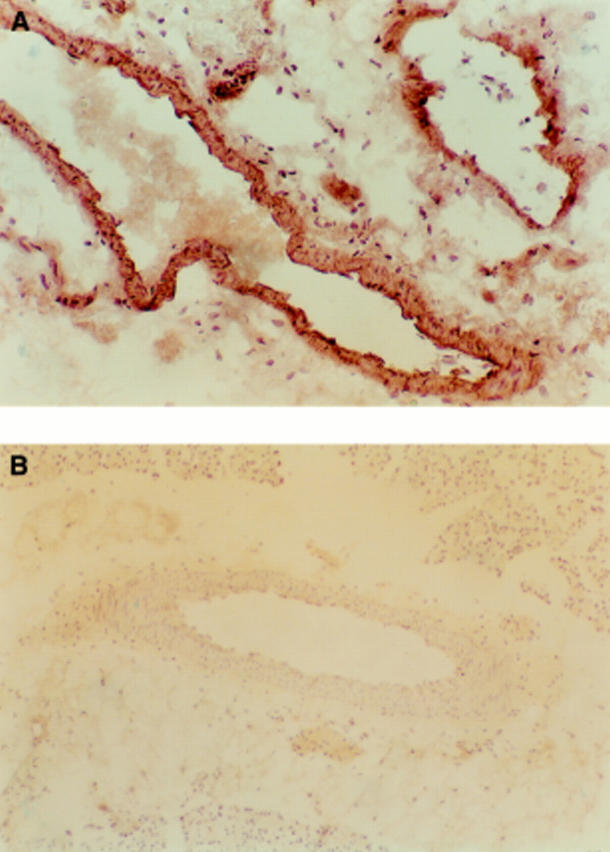
(A) Pancreatic section from the pancreatitis group showing positive staining with the antinitrotyrosine antibody. (B) Section from the control group with negative staining for the antinitrotyrosine antibody (original magnification ×200).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Shimosegawa T., Satoh A., Abe R., Kikuchi Y., Koizumi M., Toyota T. Nitric oxide modulates pancreatic edema formation in rat caerulein-induced pancreatitis. J Gastroenterol. 1995 Oct;30(5):636–642. doi: 10.1007/BF02367791. [DOI] [PubMed] [Google Scholar]
- Billiar T. R. Nitric oxide. Novel biology with clinical relevance. Ann Surg. 1995 Apr;221(4):339–349. doi: 10.1097/00000658-199504000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell R. Human responses to bacterial endotoxin. Circ Shock. 1994 Jul;43(3):137–153. [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Calver A., Collier J., Vallance P. Nitric oxide and cardiovascular control. Exp Physiol. 1993 May;78(3):303–326. doi: 10.1113/expphysiol.1993.sp003687. [DOI] [PubMed] [Google Scholar]
- Cook H. T., Bune A. J., Jansen A. S., Taylor G. M., Loi R. K., Cattell V. Cellular localization of inducible nitric oxide synthase in experimental endotoxic shock in the rat. Clin Sci (Lond) 1994 Aug;87(2):179–186. doi: 10.1042/cs0870179. [DOI] [PubMed] [Google Scholar]
- Dabrowski A., Gabryelewicz A. Nitric oxide contributes to multiorgan oxidative stress in acute experimental pancreatitis. Scand J Gastroenterol. 1994 Oct;29(10):943–948. doi: 10.3109/00365529409094868. [DOI] [PubMed] [Google Scholar]
- Davies M. G., Fulton G. J., Hagen P. O. Clinical biology of nitric oxide. Br J Surg. 1995 Dec;82(12):1598–1610. doi: 10.1002/bjs.1800821206. [DOI] [PubMed] [Google Scholar]
- Davies M. G., Hagen P. O. Systemic inflammatory response syndrome. Br J Surg. 1997 Jul;84(7):920–935. doi: 10.1002/bjs.1800840707. [DOI] [PubMed] [Google Scholar]
- Exley A. R., Leese T., Holliday M. P., Swann R. A., Cohen J. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut. 1992 Aug;33(8):1126–1128. doi: 10.1136/gut.33.8.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey T. J., 3rd, Yoshioka T., Shires G. T., Fantini G. A. The role of tumor necrosis factor and nitric oxide in the acute cardiovascular response to endotoxin. Ann Surg. 1996 Jan;223(1):63–69. doi: 10.1097/00000658-199601000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulis A. K., Murray W. R., Galloway D., McCartney A. C., Lang E., Veitch J., Whaley K. Endotoxaemia and complement activation in acute pancreatitis in man. Gut. 1982 Aug;23(8):656–661. doi: 10.1136/gut.23.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y. J., Almqvist M., Hansson G. K. cDNA cloning and expression of inducible nitric oxide synthase from rat vascular smooth muscle cells. Biochim Biophys Acta. 1994 Aug 2;1218(3):421–424. doi: 10.1016/0167-4781(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Grewal H. P., Kotb M., el Din A. M., Ohman M., Salem A., Gaber L., Gaber A. O. Induction of tumor necrosis factor in severe acute pancreatitis and its subsequent reduction after hepatic passage. Surgery. 1994 Feb;115(2):213–221. [PubMed] [Google Scholar]
- Gómez-Jiménez J., Salgado A., Mourelle M., Martín M. C., Segura R. M., Peracaula R., Moncada S. L-arginine: nitric oxide pathway in endotoxemia and human septic shock. Crit Care Med. 1995 Feb;23(2):253–258. doi: 10.1097/00003246-199502000-00009. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic Res. 1996 Jul;25(1):57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Geng Y. J., Holm J., Hårdhammar P., Wennmalm A., Jennische E. Arterial smooth muscle cells express nitric oxide synthase in response to endothelial injury. J Exp Med. 1994 Aug 1;180(2):733–738. doi: 10.1084/jem.180.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. I., Cruickshank A., Gudgeon M., Jehanli A., Shenkin A., Imrie C. W. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut. 1993 Jan;34(1):41–45. doi: 10.1136/gut.34.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H., al-Mehdi A. B. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995 May 15;364(3):279–282. doi: 10.1016/0014-5793(95)00307-u. [DOI] [PubMed] [Google Scholar]
- Kanno K., Hirata Y., Imai T., Iwashina M., Marumo F. Regulation of inducible nitric oxide synthase gene by interleukin-1 beta in rat vascular endothelial cells. Am J Physiol. 1994 Dec;267(6 Pt 2):H2318–H2324. doi: 10.1152/ajpheart.1994.267.6.H2318. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Szlachcic A., Dembinski A., Warzecha Z., Jaworek J., Stachura J. Nitric oxide in pancreatic secretion and hormone-induced pancreatitis in rats. Int J Pancreatol. 1994 Feb;15(1):19–28. doi: 10.1007/BF02924384. [DOI] [PubMed] [Google Scholar]
- Kuo P. C., Schroeder R. A. The emerging multifaceted roles of nitric oxide. Ann Surg. 1995 Mar;221(3):220–235. doi: 10.1097/00000658-199503000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusske A. M., Rongione A. J., Reber H. A. Cytokines and acute pancreatitis. Gastroenterology. 1996 Feb;110(2):639–642. doi: 10.1053/gast.1996.v110.agast960639. [DOI] [PubMed] [Google Scholar]
- Lampel M., Kern H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Liu S. F., Adcock I. M., Old R. W., Barnes P. J., Evans T. W. Differential regulation of the constitutive and inducible nitric oxide synthase mRNA by lipopolysaccharide treatment in vivo in the rat. Crit Care Med. 1996 Jul;24(7):1219–1225. doi: 10.1097/00003246-199607000-00026. [DOI] [PubMed] [Google Scholar]
- Liu X., Nakano I., Yamaguchi H., Ito T., Goto M., Koyanagi S., Kinjoh M., Nawata H. Protective effect of nitric oxide on development of acute pancreatitis in rats. Dig Dis Sci. 1995 Oct;40(10):2162–2169. doi: 10.1007/BF02209000. [DOI] [PubMed] [Google Scholar]
- Lomis T. J., Siffring C. W., Chalasani S., Ziegler D. W., Lentz K. E., Stauffer K. E., McMillan A., Agarwal N., Lowenstein C. J., Rhoads J. E., Jr First place winner of the Conrad Jobst Award in the gold medal paper competition. Nitric oxide synthase inhibitors N-monomethylarginine and aminoguanidine prevent the progressive and severe hypotension associated with a rat model of pancreatitis. Am Surg. 1995 Jan;61(1):7–10. [PubMed] [Google Scholar]
- MacNaul K. L., Hutchinson N. I. Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1330–1334. doi: 10.1006/bbrc.1993.2398. [DOI] [PubMed] [Google Scholar]
- McKay C. J., Gallagher G., Brooks B., Imrie C. W., Baxter J. N. Increased monocyte cytokine production in association with systemic complications in acute pancreatitis. Br J Surg. 1996 Jul;83(7):919–923. doi: 10.1002/bjs.1800830712. [DOI] [PubMed] [Google Scholar]
- Molero X., Guarner F., Salas A., Mourelle M., Puig V., Malagelada J. R. Nitric oxide modulates pancreatic basal secretion and response to cerulein in the rat: effects in acute pancreatitis. Gastroenterology. 1995 Jun;108(6):1855–1862. doi: 10.1016/0016-5085(95)90150-7. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Norman J. G., Fink G. W., Franz M. G. Acute pancreatitis induces intrapancreatic tumor necrosis factor gene expression. Arch Surg. 1995 Sep;130(9):966–970. doi: 10.1001/archsurg.1995.01430090052018. [DOI] [PubMed] [Google Scholar]
- Norman J., Franz M., Messina J., Riker A., Fabri P. J., Rosemurgy A. S., Gower W. R., Jr Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery. 1995 Jun;117(6):648–655. doi: 10.1016/s0039-6060(95)80008-5. [DOI] [PubMed] [Google Scholar]
- Rodeberg D. A., Chaet M. S., Bass R. C., Arkovitz M. S., Garcia V. F. Nitric oxide: an overview. Am J Surg. 1995 Sep;170(3):292–303. doi: 10.1016/s0002-9610(05)80017-0. [DOI] [PubMed] [Google Scholar]
- Sato K., Miyakawa K., Takeya M., Hattori R., Yui Y., Sunamoto M., Ichimori Y., Ushio Y., Takahashi K. Immunohistochemical expression of inducible nitric oxide synthase (iNOS) in reversible endotoxic shock studied by a novel monoclonal antibody against rat iNOS. J Leukoc Biol. 1995 Jan;57(1):36–44. [PubMed] [Google Scholar]
- Sweiry J. H., Mann G. E. Role of oxidative stress in the pathogenesis of acute pancreatitis. Scand J Gastroenterol Suppl. 1996;219:10–15. doi: 10.3109/00365529609104992. [DOI] [PubMed] [Google Scholar]
- Szabó C., Salzman A. L., Ischiropoulos H. Endotoxin triggers the expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in the rat aorta in vivo. FEBS Lett. 1995 Apr 24;363(3):235–238. doi: 10.1016/0014-5793(95)00322-z. [DOI] [PubMed] [Google Scholar]
- Viedma J. A., Pérez-Mateo M., Domínguez J. E., Carballo F. Role of interleukin-6 in acute pancreatitis. Comparison with C-reactive protein and phospholipase A. Gut. 1992 Sep;33(9):1264–1267. doi: 10.1136/gut.33.9.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert A. L., Higa E. M., Niederberger M., McMurtry I. F., Raynolds M., Schrier R. W. Expression and preferential inhibition of inducible nitric oxide synthase in aortas of endotoxemic rats. J Am Soc Nephrol. 1995 Jun;5(12):2067–2072. doi: 10.1681/ASN.V5122067. [DOI] [PubMed] [Google Scholar]
- Werner J., Rivera J., Fernandez-del Castillo C., Lewandrowski K., Adrie C., Rattner D. W., Warshaw A. L. Differing roles of nitric oxide in the pathogenesis of acute edematous versus necrotizing pancreatitis. Surgery. 1997 Jan;121(1):23–30. doi: 10.1016/s0039-6060(97)90178-1. [DOI] [PubMed] [Google Scholar]
- Windsor J. A., Fearon K. C., Ross J. A., Barclay G. R., Smyth E., Poxton I., Garden O. J., Carter D. C. Role of serum endotoxin and antiendotoxin core antibody levels in predicting the development of multiple organ failure in acute pancreatitis. Br J Surg. 1993 Aug;80(8):1042–1046. doi: 10.1002/bjs.1800800840. [DOI] [PubMed] [Google Scholar]
- de Beaux A. C., Goldie A. S., Ross J. A., Carter D. C., Fearon K. C. Serum concentrations of inflammatory mediators related to organ failure in patients with acute pancreatitis. Br J Surg. 1996 Mar;83(3):349–353. doi: 10.1002/bjs.1800830317. [DOI] [PubMed] [Google Scholar]
- van der Vliet A., Eiserich J. P., O'Neill C. A., Halliwell B., Cross C. E. Tyrosine modification by reactive nitrogen species: a closer look. Arch Biochem Biophys. 1995 Jun 1;319(2):341–349. doi: 10.1006/abbi.1995.1303. [DOI] [PubMed] [Google Scholar]