Abstract
Background—Immunoregulatory abnormalities of T cells might be of importance in the pathogenesis of pouchitis after ileoanal pouch anastomosis (IAP). Aims—To characterise T cell subsets, their state of activation, and production of cytokines in inflamed and non-inflamed pouches in patients with ulcerative colitis (UC) and familial adenomatous polyposis (FAP). The influence of T cell activation on mucosal transformation was also studied. Patients—Mucosal biopsy specimens were taken from 42 patients with IAP (33 with UC and nine with FAP). Methods—Mononuclear cells were isolated by standard techniques and characterised by three colour flow cytometry. Interferon γ (IFN-γ) production was studied using the ELISPOT technique. Results—In patients with UC with pouchitis there was a significant increase in the CD4:CD8 ratio, expression of activation markers on CD3+ cells, and number of IFNγ producing mononuclear cells compared with patients with UC without pouchitis (CD4:CD8 ratio 1.3 (range 0.7-2.7) versus 0.6 (0.1-1.0), p=0.012). In addition, a positive correlation between increased crypt depth and the number of CD4+ cells (r=0.57) was shown. Conclusion—The observed increase in activated mucosal CD4+ T cells and IFN-γ production might lead to mucosal destruction and crypt hyperplasia as seen in pouchitis.
Keywords: pouchitis; T cell activation; mucosal transformation
Full Text
The Full Text of this article is available as a PDF (168.2 KB).
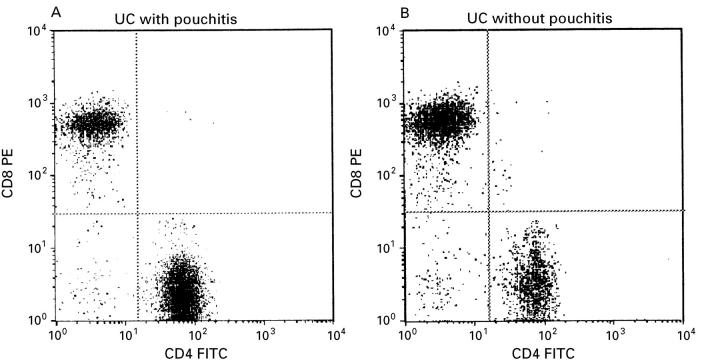
Figure 1 .
Representative example of flow cytometric analysis of CD4 and CD8 expression on CD3+ T cells on mucosal lymphocytes. Lymphocytes were isolated from inflamed pouch mucosa (A) and non-inflamed mucosa (B) of one patient with UC after treatment with metronidazole.
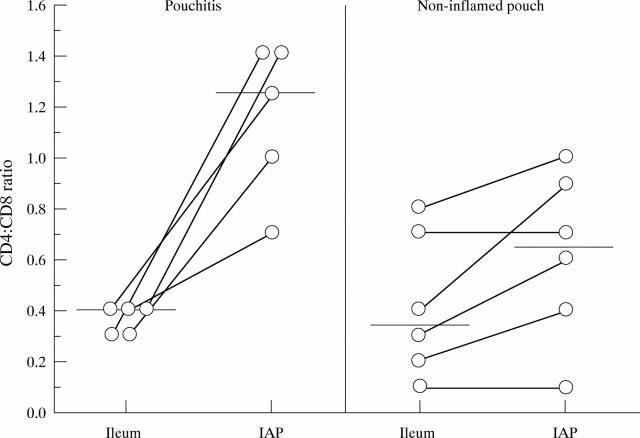
Figure 2 .
Intraindividual comparison of CD4:CD8 ratio of mucosal T cells isolated from IAP and the adjacent ileum. Bars represent the median of all patients; lines connect the intraindividual values of the CD4:CD8 ratio.
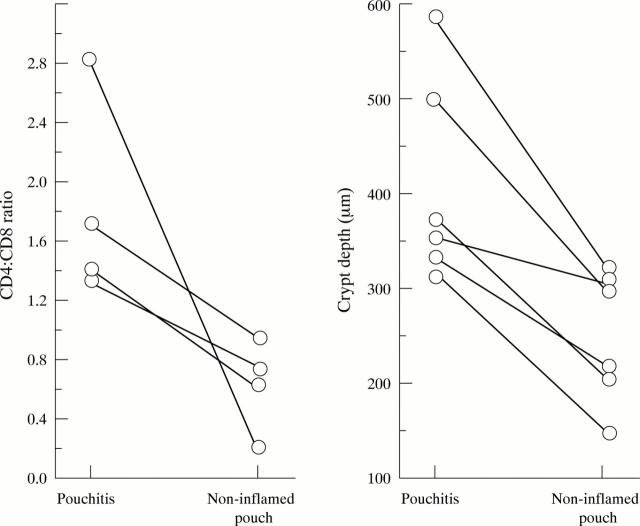
Figure 3 .
CD4:CD8 ratio and crypt lengths in individual patients during and after pouchitis. Lines connect the intraindividual values for the CD4:CD8 ratio and crypt depth measured in biopsy specimens obtained from the same patient during acute pouchitis and after treatment with normalisation of clinical and endoscopic signs of inflammation.
Figure 4 .
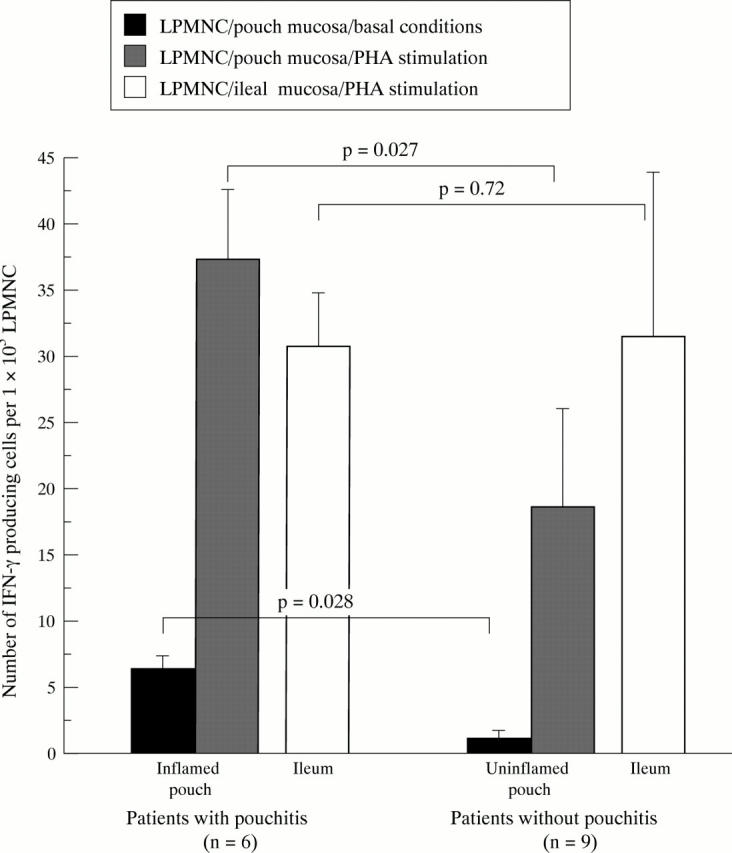
IFN-γ production in LPMNCs of patients with UC with and without pouchitis.
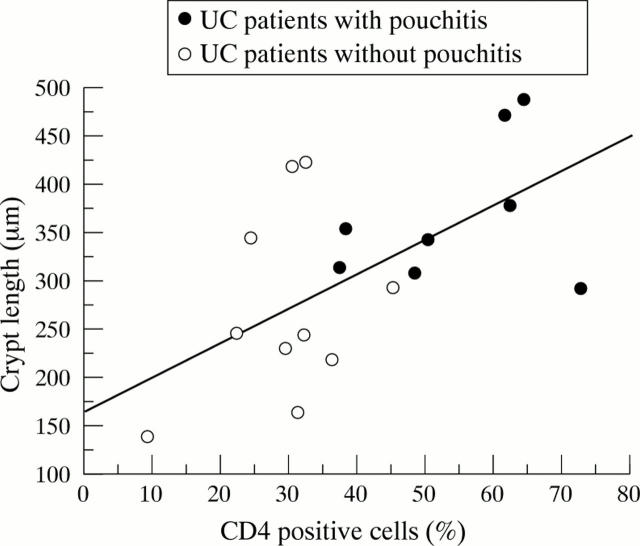
Figure 5 .
Correlation between the percentage of mucosal CD4+ T cells in pouch mucosa and the crypt depth in biopsy specimens from patients with UC and ileoanal pouches.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abreu-Martin M. T., Vidrich A., Lynch D. H., Targan S. R. Divergent induction of apoptosis and IL-8 secretion in HT-29 cells in response to TNF-alpha and ligation of Fas antigen. J Immunol. 1995 Nov 1;155(9):4147–4154. [PubMed] [Google Scholar]
- Apel R., Cohen Z., Andrews C. W., Jr, McLeod R., Steinhart H., Odze R. D. Prospective evaluation of early morphological changes in pelvic ileal pouches. Gastroenterology. 1994 Aug;107(2):435–443. doi: 10.1016/0016-5085(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Clarke R. M. Mucosal architecture and epithelial cell production rate in the small intestine of the albino rat. J Anat. 1970 Nov;107(Pt 3):519–529. [PMC free article] [PubMed] [Google Scholar]
- Creemers P. C. Determination of co-expression of activation antigens on proliferating CD4+, CD4+ CD8+ and CD8+ lymphocyte subsets by dual parameter flow cytometry. J Immunol Methods. 1987 Mar 12;97(2):165–171. doi: 10.1016/0022-1759(87)90456-x. [DOI] [PubMed] [Google Scholar]
- Dijkmans R., Billiau A. Interferon gamma: a master key in the immune system. Curr Opin Immunol. 1988 Dec;1(2):269–274. doi: 10.1016/0952-7915(88)90013-1. [DOI] [PubMed] [Google Scholar]
- Fink S., de la Barrera S., Minnucci F., Valdez R., Baliña L. M., Sasiain M. C. IFN-gamma, IL-6 and IL-4 modulate M. leprae- or PPD-specific cytotoxic T cells in leprosy patients. Scand J Immunol. 1993 Dec;38(6):551–558. doi: 10.1111/j.1365-3083.1993.tb03240.x. [DOI] [PubMed] [Google Scholar]
- Gionchetti P., Campieri M., Belluzzi A., Bertinelli E., Ferretti M., Brignola C., Poggioli G., Miglioli M., Barbara L. Mucosal concentrations of interleukin-1 beta, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in pelvic ileal pouches. Dig Dis Sci. 1994 Jul;39(7):1525–1531. doi: 10.1007/BF02088059. [DOI] [PubMed] [Google Scholar]
- Goldberg P. A., Herbst F., Beckett C. G., Martelli B., Kontakou M., Talbot I. C., Ciclitira P. J., Nicholls R. J. Leucocyte typing, cytokine expression, and epithelial turnover in the ileal pouch in patients with ulcerative colitis and familial adenomatous polyposis. Gut. 1996 Apr;38(4):549–553. doi: 10.1136/gut.38.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths C. E., Barrison I. G., Leonard J. N., Caun K., Valdimarsson H., Fry L. Preferential activation of CD4 T lymphocytes in the lamina propria of gluten-sensitive enteropathy. Clin Exp Immunol. 1988 May;72(2):280–283. [PMC free article] [PubMed] [Google Scholar]
- Handelsman J. C., Fishbein R. H., Hoover H. C., Jr, Smith G. W., Haller J. A., Jr Endorectal pull-through operation in adults after colectomy and excision of rectal mucosa. Surgery. 1983 Feb;93(2):247–253. [PubMed] [Google Scholar]
- Hirata I., Berrebi G., Austin L. L., Keren D. F., Dobbins W. O., 3rd Immunohistological characterization of intraepithelial and lamina propria lymphocytes in control ileum and colon and in inflammatory bowel disease. Dig Dis Sci. 1986 Jun;31(6):593–603. doi: 10.1007/BF01318690. [DOI] [PubMed] [Google Scholar]
- James S. P., Fiocchi C., Graeff A. S., Strober W. Phenotypic analysis of lamina propria lymphocytes. Predominance of helper-inducer and cytolytic T-cell phenotypes and deficiency of suppressor-inducer phenotypes in Crohn's disease and control patients. Gastroenterology. 1986 Dec;91(6):1483–1489. [PubMed] [Google Scholar]
- Kock N. G., Darle N., Hultén L., Kewenter J., Myrvold H., Philipson B. Ileostomy. Curr Probl Surg. 1977 Aug;14(8):1–52. doi: 10.1016/s0011-3840(77)80065-8. [DOI] [PubMed] [Google Scholar]
- Lionetti P., Breese E., Braegger C. P., Murch S. H., Taylor J., MacDonald T. T. T-cell activation can induce either mucosal destruction or adaptation in cultured human fetal small intestine. Gastroenterology. 1993 Aug;105(2):373–381. doi: 10.1016/0016-5085(93)90710-t. [DOI] [PubMed] [Google Scholar]
- Luukkonen P., Järvinen H., Tanskanen M., Kahri A. Pouchitis--recurrence of the inflammatory bowel disease? Gut. 1994 Feb;35(2):243–246. doi: 10.1136/gut.35.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988 Apr 1;167(4):1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Patel S., Gionchetti P., Vaux D., Jewell D. P. Macrophage subpopulations in lamina propria of normal and inflamed colon and terminal ileum. Gut. 1989 Jun;30(6):826–834. doi: 10.1136/gut.30.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwissen S. G., Hoitsma H., Boot H., Seldenrijk C. A. Pouchitis (pouch ileitis). Neth J Med. 1989 Jun;35 (Suppl 1):S54–S66. [PubMed] [Google Scholar]
- Parks A. G., Nicholls R. J. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978 Jul 8;2(6130):85–88. doi: 10.1136/bmj.2.6130.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. T., Bain I., Youngs D., Keighley M. R. Cytokine production in pouchitis is similar to that in ulcerative colitis. Dis Colon Rectum. 1995 Aug;38(8):831–837. doi: 10.1007/BF02049839. [DOI] [PubMed] [Google Scholar]
- Pender S. L., Lionetti P., Murch S. H., Wathan N., MacDonald T. T. Proteolytic degradation of intestinal mucosal extracellular matrix after lamina propria T cell activation. Gut. 1996 Aug;39(2):284–290. doi: 10.1136/gut.39.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirzer U., Schönhaar A., Fleischer B., Hermann E., Meyer zum Büschenfelde K. H. Reactivity of infiltrating T lymphocytes with microbial antigens in Crohn's disease. Lancet. 1991 Nov 16;338(8777):1238–1239. doi: 10.1016/0140-6736(91)92104-a. [DOI] [PubMed] [Google Scholar]
- Qiao L., Schürmann G., Betzler M., Meuer S. C. Activation and signaling status of human lamina propria T lymphocytes. Gastroenterology. 1991 Dec;101(6):1529–1536. doi: 10.1016/0016-5085(91)90388-2. [DOI] [PubMed] [Google Scholar]
- Riecken E. O., Stallmach A., Zeitz M., Schulzke J. D., Menge H., Gregor M. Growth and transformation of the small intestinal mucosa--importance of connective tissue, gut associated lymphoid tissue and gastrointestinal regulatory peptides. Gut. 1989 Nov;30(11):1630–1640. doi: 10.1136/gut.30.11.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn W. J. Pouchitis following ileal pouch-anal anastomosis: definition, pathogenesis, and treatment. Gastroenterology. 1994 Dec;107(6):1856–1860. doi: 10.1016/0016-5085(94)90832-x. [DOI] [PubMed] [Google Scholar]
- Schmitt-Gräff A., Hummel M., Zemlin M., Schneider T., Ullrich R., Heise W., Zeitz M., Riecken E. O., Stein H. Intestinal T-cell lymphoma: a reassessment of cytomorphological and phenotypic features in relation to patterns of small bowel remodelling. Virchows Arch. 1996 Sep;429(1):27–36. doi: 10.1007/BF00196817. [DOI] [PubMed] [Google Scholar]
- Schneider T., Ullrich R., Bergs C., Schmidt W., Riecken E. O., Zeitz M. Abnormalities in subset distribution, activation, and differentiation of T cells isolated from large intestine biopsies in HIV infection. The Berlin Diarrhoea/Wasting Syndrome Study Group. Clin Exp Immunol. 1994 Mar;95(3):430–435. doi: 10.1111/j.1365-2249.1994.tb07014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., MacDermott R. P., Raedler A., Pinnau R., Bertovich M. J., Nash G. S. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991 Oct;101(4):1020–1030. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Feb 25;57(1-3):301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- Shepherd N. A., Jass J. R., Duval I., Moskowitz R. L., Nicholls R. J., Morson B. C. Restorative proctocolectomy with ileal reservoir: pathological and histochemical study of mucosal biopsy specimens. J Clin Pathol. 1987 Jun;40(6):601–607. doi: 10.1136/jcp.40.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. A., Dozois R. R. The J ileal pouch-anal anastomosis. World J Surg. 1987 Dec;11(6):727–734. doi: 10.1007/BF01656595. [DOI] [PubMed] [Google Scholar]
- Ullrich R., Heise W., Bergs C., L'age M., Riecken E. O., Zeitz M. Effects of zidovudine treatment on the small intestinal mucosa in patients infected with the human immunodeficiency virus. Gastroenterology. 1992 May;102(5):1483–1492. doi: 10.1016/0016-5085(92)91705-9. [DOI] [PubMed] [Google Scholar]
- Ullrich R., Schneider T., Schieferdecker H. L., Jahn H. U., Riecken E. O., Zeitz M. Cell activation and proliferation in the large intestine of patients with Crohn's disease or ulcerative colitis and controls. Adv Exp Med Biol. 1995;371B:1281–1282. [PubMed] [Google Scholar]
- Ullrich R., Zeitz M., Heise W., L'age M., Ziegler K., Bergs C., Riecken E. O. Mucosal atrophy is associated with loss of activated T cells in the duodenal mucosa of human immunodeficiency virus (HIV)-infected patients. Digestion. 1990;46 (Suppl 2):302–307. doi: 10.1159/000200401. [DOI] [PubMed] [Google Scholar]
- Van der Meide P. H., Dubbeld M., Schellekens H. Monoclonal antibodies to human immune interferon and their use in a sensitive solid-phase ELISA. J Immunol Methods. 1985 May 23;79(2):293–305. doi: 10.1016/0022-1759(85)90109-7. [DOI] [PubMed] [Google Scholar]
- de Silva H. J., Gatter K. C., Millard P. R., Kettlewell M., Mortensen N. J., Jewell D. P. Crypt cell proliferation and HLA-DR expression in pelvic ileal pouches. J Clin Pathol. 1990 Oct;43(10):824–828. doi: 10.1136/jcp.43.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva H. J., Jones M., Prince C., Kettlewell M., Mortensen N. J., Jewell D. P. Lymphocyte and macrophage subpopulations in pelvic ileal pouches. Gut. 1991 Oct;32(10):1160–1165. doi: 10.1136/gut.32.10.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spreeuwel J. P., Lindeman J., Meijer C. J. A quantitative study of immunoglobulin containing cells in the differential diagnosis of acute colitis. J Clin Pathol. 1985 Jul;38(7):774–777. doi: 10.1136/jcp.38.7.774. [DOI] [PMC free article] [PubMed] [Google Scholar]